MOLECULAR WEIGHT(syn. molecular mass) - the mass of a molecule of a substance, expressed in carbon units of atomic mass (a carbon unit of atomic mass is 1/12 of the mass of an atom of the carbon isotope 12 C); along with atomic masses, it serves as the basis for all kinds of calculations performed using chemistry. formulas and equations, including calculations made in biochemistry. and clinical diagnostic laboratories.
If the chem. formula of a substance, then its M. v. can be calculated as the sum of the atomic weights (mass) of chemical atoms. elements (see atomic weight) that make up the molecule of this substance. For example, M. v. carbon dioxide(CO 2) is equal to:
12,011 + 2 * 15,9994 = 44,0098.
For substances in a gaseous or dissolved state, experimental methods definitions of M. v. most justified. M.v. (M1) of a gas is usually determined by measuring it relative density D for gas, M.v. which (M2) is known; then M1 = M2*D. M.v. gas can also be determined if its normal density d is known, i.e. the mass of 1 liter of gas in grams at a pressure of 760 mm Hg. Art. and 0 °C. In this case, M. v. gas is equal to M = 22.42*d.
To determine M. century. dissolved substance in such a solvent, in which this substance does not undergo dissociation or association, the decrease in the freezing point of the solution Δt (see Cryometry) observed during dissolution is most often measured A g of test substance in b g of solvent: M = (K*a*1000)/(Δt*b), where K is the cryometric (cryoscopic) constant of the solvent.
M.v. dissolved substance can also be determined by measuring the osmotic pressure of the solution (see Osmotic pressure). In this case, M = (m*R*T)/p, where m is the mass of the dissolved substance in grams contained in 1 liter of solution, p is the osmotic pressure in atm, T is the temperature in degrees Kelvin and R is the gas temperature constant in l*atm/mol*deg. This method is successfully used to determine M. century. proteins, polysaccharides, nucleic and other high-molecular compounds (see). M.v. proteins and other biopolymers can be determined by ultracentrifugation (see).
In practice, biochemistry, wedge, and sanitary hygiene. laboratories to perform various kinds In calculations, a unit of quantity of a substance called a mole is also widely used.
A mole is an amount of substance containing so many molecules, atoms, ions, electrons or other structural units, how many atoms are contained in 12 g of the carbon isotope 12 C. The number of molecules, atoms or other structural units contained in one mole of any substance, called Avogadro’s number, is determined with great accuracy. For practical calculations it is taken equal to
6.023*10 23 mol -1.
The mass of one mole of a substance, expressed in grams, is numerically equal to M.v. substance is called molar mass, or gram-molecule.
Bibliography: Belki, ed. G. Neurath and K. Bailey, trans. from English, vol. 2, p. 276, M., 195 6: Gaurowitz F. Chemistry and function of proteins, trans. from English, M., 1965; Ostwald-Luther - Drucker, Physicochemical measurements, trans. with German, part 1, €. 294, L., 1935.
We see that one weight significantly outweighs seven plastic balls. Experience with scales gives us the answer - there is more substance in an iron weight, this is if we compare masses - measures of inertia of iron and plastic.
But what if we compare not the masses, but the amount of substance that went into making the balls and weights, in fact, the number of particles of which they are composed? Taking the balls and the weight in our hands, we will see that the weight is actually lost against the background of these balls. If we could count the number of particles that are included in iron and plastic, then we would see that the number of iron atoms would be significantly less quantity molecules in all plastic balls. This means there is more substance in plastic.
Both answers are correct.
The thing is that in the first case we compared mass, that is, a measure of the inertia of bodies, and in the second case we compared the number of molecules, the amount of substance.
We can draw a simple analogy with sugar in a measuring cup. The question of how much sugar there is can be answered by looking at the division of the glass and approximately telling how many grams of sugar there are. You can count each grain in the glass and answer how many of them the glass contains. Both the first and second answers will be correct. When is it more convenient to talk about the mass of molecules, and when is it more convenient to talk about the amount of substance? This is precisely the topic of the lesson: “Mass of molecules, Amount of substance.”
In the 19th century, the Italian scientist Avogadro established interesting fact: if two different gases, for example hydrogen and oxygen, are in the same vessels, at the same pressures and temperatures, then in each vessel there will be the same number of molecules, although the masses of the gases can differ very much, in our example - 16 times (Fig. 2 ).
Rice. 2. Avogadro's experiment ()
All this means that some properties of a body are determined precisely by the number of molecules, and not just by mass.
What do we mean by the term “amount of substance”? Any substance consists of molecules, atoms, ions - which means that by the amount of a substance we understand the number of molecules.
Physical quantity that determines the number of molecules in this body, called amount of substance. Designated Greek letterν - nude.
We agreed to take as a unit amount of a substance the quantity that contains as many particles (atoms, molecules) as there are atoms in 0.012 kg (12 grams) of a carbon isotope with atomic mass 12.
This unit is called mole.
From this definition it follows that in one mole of any substance there will be the same number of molecules. One mole of any substance contains 6.02 10 23 molecules or particles. This quantity is called Avogadro's constant.

Rice. 3. Determination of the total number of molecules ()
This formula allows you to find out full number molecules at known quantity substances.
The mass of the molecule is extremely small. Physicists determined this using a so-called mass spectrograph. For example, the value of the mass of a water molecule (Fig. 4):

Rice. 4. Determination of the mass of a water molecule ()
As we see, just as in cases with the amount of a substance, comparing the mass of one molecule with a mass standard, a kilogram, is not very convenient. If in cases with the amount of substance the numbers are huge, then in cases with the mass of molecules the numbers are very small. That is why a special extra-systemic unit was chosen as a unit of measurement for the mass of a molecule or atom - atomic mass unit. We will compare a unit of mass not with a standard, but with the mass of a molecule of some substance.
This substance became the most common element in nature - carbon, which is included in everything. organic compounds. The atomic mass unit is equal to:
1 amu = 1/12 mass of carbon - 12 (isotope with 12 nucleons)
1 amu = 1.66·10 -27 kg
Since we will measure the mass of molecules in atomic units masses, then we come to a new physical quantity- relative molecular weight.
The ratio of the mass of a molecule (atom) of a given substance to 1/12 of the mass of a carbon atom is called relative molecular weight(or relative atomic mass) in the case of the atomic structure of a substance.
Formulas expressing this definition:
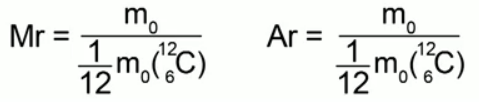
Relative molecular weight is a dimensionless quantity; it is not measured in anything. Nothing prevents us from continuing to measure the masses of atoms and molecules in kilograms whenever it is convenient for us. From the chemistry course we know that: the relative molecular mass of a substance is equal to the sum of the relative atomic masses of the elements included in it. For example, for water H2O the relative molecular weight will be:
Mr = 1 2 + 16 = 18
Sum relative molecular weight oxygen (16) and two hydrogens (2.1) will give 18
How to find the commonality between the mass in kilograms and the amount of substance in moles? This quantity is molar mass.
Molar mass is the mass of one mole of a substance.
Designated [M], measured in kg/mol.
Molar mass is equal to the ratio of mass to amount of substance:
We obtain formulas that relate various characteristics of molecules.
For determining molar mass chemical element let's turn to periodic table chemical elements of Mendeleev - we simply take atomic mass A (the number of nucleons of the required element) - this will be its molar mass, expressed in g/mol.
For example, for aluminum (Fig. 5):

Rice. 5. Determination of the molar mass of a substance ( )
The atomic mass of aluminum will be 27 and the molar mass will be 0.027 kg/mol.
This is explained by the fact that the molar mass of carbon is 12 g/mol by definition, while the nucleus of a carbon atom contains 12 nucleons - 6 protons and 6 neutrons, it turns out that each nucleon contributes 1 g/mol to the molar mass, so the molar mass of a chemical element with atomic mass A will be equal to A g/mol.
The molar mass of a substance whose molecule consists of several atoms is obtained by simply summing the molar masses, for example (Fig. 6):

Rice. 6. Molar mass of carbon dioxide ()
You need to be especially careful with the molar masses of some gases, such as hydrogen gas, nitrogen, oxygen - their molecule consists of two atoms - H 2, N 2, O 2, and helium, often found in problems, is monatomic and has a molecular weight of 4 g / mol, prescribed by the periodic table (Fig. 7).

Rice. 7. Molar masses of some gases ()
One mole of any substance contains the Avogadro number of molecules, which means that if we multiply the Avogadro number (the number of molecules in one mole) by the mass of one molecule m0, then we get the molar mass of the substance, that is, the mass of one mole of the substance:
M = m 0 N A
If 25 students are studying in a classroom with an area of 50 m2, then for each student there is 2 m2. When they go to a gym with an area of 500 m2, each student will already have 20 m2. The number of students has not changed, but they have become less distributed, in this case they say: the concentration of people has decreased. In the same way, the concept of concentration is introduced for molecules in molecular kinetic theory.
Concentration(n) is the number of molecules per unit volume of a substance. It is equal to the ratio of the number of molecules to volume:
Formulas relating concentration to other characteristics of molecules:

Using these formulas, we can compare substances both by the number of molecules and by mass.
We have received everything we need to build a molecular kinetic theory, which we will do in the next lessons.
Bibliography
- Tikhomirova S.A., Yavorsky B.M. Physics ( a basic level of) - M.: Mnemosyne, 2012.
- Gendenshtein L.E., Dick Yu.I. Physics 10th grade. - M.: Mnemosyne, 2014.
- Kikoin I.K., Kikoin A.K. Physics - 9, Moscow, Education, 1990.
- Lib.podelise.ru ().
- Class-fizika.spb.ru ().
- Bolshoyvopros.ru ().
Homework
- Define the amount of a substance.
- Name the unit of measurement for the mass of a molecule or atom.
- Define relative molecular weight.


Basic provisions of the ICT. Mass and size of molecules. Amount of substance. Molecular physics
MKT is easy!
“Nothing exists except atoms and empty space...” - Democritus
“Any body can divide indefinitely” - Aristotle
Basic principles of molecular kinetic theory (MKT)
Purpose of the ICT- this is an explanation of the structure and properties of various macroscopic bodies and the thermal phenomena that occur in them, by the movement and interaction of the particles that make up the bodies.
Macroscopic bodies- This big bodies consisting of a huge number of molecules.
Thermal phenomena- phenomena associated with heating and cooling of bodies.
Main statements of the ICT
1. Matter consists of particles (molecules and atoms).
2. There are gaps between the particles.
3. Particles move randomly and continuously.
4. Particles interact with each other (attract and repel).
MKT confirmation:
1. experimental
- mechanical crushing of a substance; dissolving a substance in water; compression and expansion of gases; evaporation; deformation of bodies; diffusion; Brigman's experiment: oil is poured into a vessel, a piston presses on top of the oil, at a pressure of 10,000 atm, oil begins to seep through the walls of the steel vessel;
Diffusion; Brownian motion of particles in a liquid under the impacts of molecules;
Poor compressibility of solids and liquid bodies; significant effort to break solids; merging of liquid droplets;
2. direct
- photography, determination of particle sizes. 
Brownian motion
Brownian motion is the thermal movement of suspended particles in a liquid (or gas).
Brownian motion has become evidence of the continuous and chaotic (thermal) movement of the molecules of matter.
- discovered by the English botanist R. Brown in 1827
- a theoretical explanation based on MCT was given by A. Einstein in 1905.
- experimentally confirmed by the French physicist J. Perrin.
Mass and size of molecules
Particle sizes
The diameter of any atom is about cm.

Number of molecules in a substance
where V is the volume of the substance, Vo is the volume of one molecule
Mass of one molecule
where m is the mass of the substance,
N - number of molecules in a substance
SI unit of mass: [m]= 1 kg
IN atomic physics mass is usually measured in atomic mass units (amu).
Conventionally, it is considered to be 1 amu. :
Relative molecular weight of the substance
For convenience of calculations, a quantity is introduced - the relative molecular mass of the substance.
The mass of a molecule of any substance can be compared to 1/12 the mass of a carbon molecule.
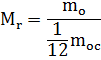
where the numerator is the mass of the molecule and the denominator is 1/12 the mass of the carbon atom
This is a dimensionless quantity, i.e. has no units of measurement
Relative atomic mass of a chemical element
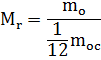
where the numerator is the mass of the atom and the denominator is 1/12 the mass of the carbon atom
The quantity is dimensionless, i.e. has no units of measurement
The relative atomic mass of each chemical element is given in the periodic table.
Another way to determine the relative molecular mass of a substance
The relative molecular mass of a substance is equal to the sum of the relative atomic masses of the chemical elements that make up the molecule of the substance.
We take the relative atomic mass of any chemical element from the periodic table!)
Quantity of substance
The amount of substance (ν) determines the relative number of molecules in the body.
where N is the number of molecules in the body, and Na is Avogadro's constant
Unit of measurement of the amount of substance in the SI system: [ν]= 1 mol
1 mole- this is the amount of substance that contains as many molecules (or atoms) as there are atoms in carbon weighing 0.012 kg.
Remember!
1 mole of any substance contains same number atoms or molecules!
But!
Same amounts of substance for different substances have different masses!
Avogadro's constant
The number of atoms in 1 mole of any substance is called Avogadro's number or Avogadro's constant:
Molar mass
Molar mass (M) is the mass of a substance taken in one mole, or otherwise, it is the mass of one mole of a substance.
Molecule mass
- Avogadro's constant
Unit of molar mass: [M]=1 kg/mol.
Formulas for solving problems
These formulas are obtained by substituting the above formulas.
Mass of any amount of substance
The most important method for determining the molecular weights of gaseous substances is based on Avogadro's law. But before talking about this method, it should be recalled in what units molecular and atomic weights.
When calculating atomic weights, the weight of the hydrogen atom, as the lightest element, was initially taken as one, and the atomic weights of other elements were calculated in relation to it. But since for most elements the atomic weights are determined from their oxygen compounds, in fact, the calculations were made in relation to the atomic weight of oxygen, which was considered equal to 16. The ratio between the atomic weights of oxygen and hydrogen was assumed to be 16:1. Subsequently more precise research showed that this ratio is 15.88:1, or 16:1.008. Therefore, if we assume the atomic weight of hydrogen to be 1, the atomic weight of oxygen will be 15.88. For practical reasons, it was decided to leave the atomic weight of oxygen at 16, taking the atomic weight of 1.008 for hydrogen.
Thus, the current unit of atomic weight is 1/16th the weight of an oxygen atom. This unit is called the “oxygen unit”. Hydrogen atom weightis equal to 1.008 oxygen units, the weight of a sulfur atom is 32.06 oxygen units, etc.
Atomic weight element called weight his atom, heightmarried V oxygen units.
Since the weight of a molecule of any equal to the sum weights of the atoms that form it, it is clear that molecular weights must be expressed in the same units as atomic weights. For example, the weight of a hydrogen molecule consisting of two atoms is equal to 2.016 oxygen units; the weight of an oxygen molecule, also consisting of two atoms, is equal to 32 oxygen units; the weight of a water molecule containing two hydrogen atoms and one oxygen atom is 16 + 2.016 = 18.016 oxygen units, etc.
Molecular weight simple or complex namesvaries weight his molecules, expressed V oxygen units.
Let's now see how the molecular weights of gaseous substances are determined.
According to Avogadro's law, equal volumes of gases taken at the same pressure and the same temperature contain equal number molecules. It follows that the weights equal volumes two gases must relate to each other as their molecular weights.
Let's take, for example, one liter of two different gases. Let each of them contain N molecules. Let us denote the weight of a liter of the first gas by g, and the second through g 1. Let us denote the molecular weights of gases, respectively, by M and M 1. Since the weight of a liter of gas is equal to the sum of the weights of the molecules in it,
g = N M And g 1 =N M 1 Dividing the first equality by the second, we get: (1)
The ratio of the weight of a given gas to the weight of the same volume of another gas taken at the same temperature and the same pressure is called the density of the first gas relative to the second. For example, 1 l carbon dioxide weighs 1.98 g, and 1 l hydrogen under the same conditions is 0.09 g, from which the density of carbon dioxide by hydrogen will be 1.98: 0.09 = 22.
Denoting the gas density with the letter D, Let's rewrite equation (1):
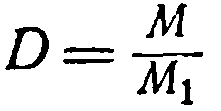
where
M = D M 1 (2)
Molecular weight gas equals his density By attitude to another gas, multiplied on molecular weight second gas.
Very often, the densities of various gases are determined in relation to hydrogen as the lightest of all cans. Since the molecular weight of hydrogen itself is 2.016, in this case the formula for calculating molecular weights takes the form:
M = 2.016 D
or, if we round the molecular weight of hydrogen to 2:
M = 2 D
Calculating, for example, using this formula the molecular weight of carbon dioxide, the density of which for hydrogen, as indicated above, is 22, we find:
M = 2 22 = 44
The molecular weight of a gas is often also calculated based on its density in air. Although air is a mixture of several gases, we can still talk about the average molecular weight of air, determined from the density of air in terms of hydrogen. The molecular weight of air found in this way is 29.
Denoting the density of the gas under study in air through D 1, we obtain the following formula to calculate molecular weights:
M = 29 D 1
The number 29 is useful to remember, as it is often used in calculations.
In practice, determining molecular weight comes down to measuring the weight and volume of a certain amount of the gas under study and subsequent calculation of its density, after which the molecular weight is found directly from the formula. The density of a gas can be calculated in relation to any other gas whose molecular weight and weight per unit volume are known. But since the reference books indicate the weights of gases under normal conditions, and in experience it is usually necessary to measure the weight and volume of the gas under study under other conditions, then to calculate the gas density it is necessary to first bring the measured volume of gas to normal conditions (0° and 760 mm pressure).
Reduction to normal conditions is carried out on the basis of an equation combining gas laws Boyle-Mariotte and Gay-Lussac:

Where R and υ - pressure and volume of gas under experimental conditions, respectively; P 0 — normal blood pressure; υ 0 - volume of gas under normal conditions; T - absolute temperature gas.
