VACUOLES OF PLANT CELLS
Organelles of general importance, having a single-membrane structure and occurring only in plant cells.
Size and Quantity: determined by the age of the cell. In young cells, vacuoles arise from small vesicles that break off from the EPS. As cells grow and differentiate, small vacuoles merge with each other and form one or more large vacuoles, occupying up to 80% of the volume of the entire cell. As a result, the cytoplasm with the nucleus and organelles is pushed to the periphery.
Structure: central vacuoles are separated from the cytoplasm by a single membrane - tonoplast, which is similar in thickness to the plasmalemma. The vacuole cavity is filled cell sap. The composition of cell sap includes inorganic salts, sugars, organic acids and their salts, other low molecular weight substances, as well as some high molecular weight compounds (for example, proteins).
Functions: 1) osmoregulation: due to the semi-permeability of the tonoplast and plasmalemma, the corresponding molecular concentration of cell sap is maintained, i.e. the vacuole functions as an osmometer;
2) excretory: all water-soluble metabolic products (alkaloids - nicotine, caffeine; polyphenols) can be removed through the tonoplast;
3) storing: phosphates K + , Na + , Ca 2+ , salts of organic acids (oxalates, citrates, etc.), sugars and proteins accumulate in the cell sap.
RIBOSOMES
An organelle that does not have a membrane structure. It is the only organelle of general importance that is present in the cells of both prokaryotes and eukaryotes. Ribosomes were first described in 1955 G. J. Palade (Palade granules), who proved that they are ribonucleoprotein complexes (RNP).
Chemical nature: RNP= rRNA+protein.
Ribosomes account for 85% RNA, presented in a cell.
Form: The ribosome has a mushroom shape because it consists of two subunits: big And small, between them is located functional center of the ribosome (FCR) , in which, during protein biosynthesis (translation period), mRNA is located in its two triplets and an enzymatic complex operates, ensuring the assembly of a protein molecule from amino acids.
Dimensions: 15 – 35 nm. The size of a complete ribosome in prokaryotic cells is 20x17x17 nm, in eukaryotic cells - 25x20x20 nm.
Place of education: the formation of ribosomal subunits occurs in the nucleoli of the nucleus. The assembly of subunits into a complete ribosome occurs in the cytoplasm when the concentration of magnesium ions (Mg 2+) reaches 0.001 M; if this concentration decreases, dissociation of the subunits occurs. When the Mg 2+ concentration increases tenfold, reaching a value of 0.01 M, the two ribosomes interact with each other, forming a dimer.
Vacuoles and cell sap
Most mature plant cells are characterized by a large central vacuole, occupying up to 70-90% of the cell volume. In this case, the protoplast with all the organelles is located in the form of a very thin wall layer lining the cell wall. Small cytoplasmic vacuoles are usually found in the wall protoplast. Sometimes the nucleus is located in the center of the cell in nuclear pocket cytoplasm, which is connected to the wall layer by the thinnest cytoplasmic strands crossing the central vacuole.
Cell sap is an aqueous solution of various substances that are products of the vital activity of the protoplast, mainly reserve substances and waste. The reaction of cell sap is usually slightly acidic or neutral, less often alkaline. The substances that make up the cell sap are extremely diverse. These are carbohydrates, proteins, organic acids and their salts, amino acids, mineral ions, alkaloids, glycosides, tannins, pigments and other water-soluble compounds. Most of them belong to the group of ergastic substances - products of protoplast metabolism, which can appear and disappear at different periods of cell life. Many cell sap substances are formed only in plant cells.
Carbohydrates in plant cell sap are represented by monosaccharides (glucose, fructose), disaccharides (sucrose) and polysaccharides (mucus, inulin).
Glucose (grape sugar) and fructose (fruit sugar) accumulate in large quantities in juicy fruits. Sucrose (beet sugar) accumulates in large quantities in sugar beet roots and sugar cane stalks. A number of plant families (cactaceae, Crassulaceae, orchids) are characterized by the accumulation of mucus in the cell sap, which retains water. Inulin is a reserve polysaccharide, deposited as a colloidal solution in the cell sap of the underground organs of Asteraceae instead of starch.
Proteins accumulate in the form of a colloidal solution in the vacuoles of cells of ripening seeds. When seeds are dehydrated in the later stages of their development, water is removed from the vacuoles, the protein concentration in the cell sap increases, and it turns into a solid gel state. Dehydrated vacuoles of mature seeds are called aleurone grains.
Of the organic acids in cell sap, the most common are citric, malic, succinic and oxalic. These acids are found in large quantities in the cell sap of unripe fruits, giving them a sour taste. When fruits ripen, organic acids can be used as respiration substrates, so the sour taste of the fruit usually disappears. Salts of organic acids, together with mineral ions, play an important role in osmotic processes.
Tannins (tannins) are polymeric phenolic compounds with an astringent taste. They have antiseptic properties and protect plant tissues from infections and decay. The cells of the bark of stems and roots (oak, willow), unripe fruits (walnuts), leaves (tea) and some pathological growths - galls are especially rich in tannins. Tannids are used in medicine, for tanning leather, and dyeing fabrics dark brown.
Alkaloids are chemically diverse nitrogen-containing organic substances that have a bitter taste. They have the properties of bases and are found in cell sap, usually in the form of salts. Many alkaloid-bearing plants are poisonous and are not eaten by herbivores. In cells containing alkaloids, spores and germs of microorganisms do not develop, and plants are not affected by fungal and bacterial diseases. Representatives of the families Solanaceae, Poppy, Rubiaceae, Ranunculaceae, etc. are especially rich in alkaloids.
Glycosides are a large group of natural substances, compounds of sugars with alcohols, aldehydes, phenols and other substances. A number of plant glycosides are used in medicine. Glycosides also include cell sap pigments - flavonoids. One of them - anthocyanins– give cell sap a red, blue or purple color; other - flavones- yellow. Anthocyanins are associated with the color of flowers of many plants. The color scheme is determined by the reaction of the cell sap: if it is acidic, then red tones predominate, if it is neutral, purple tones predominate, and with a slightly alkaline reaction, blue tones predominate. The appearance of shades is also influenced by the formation of anthocyanin complexes with various metals. Flavones are responsible for the yellow color of the petals of a number of plants.
The importance of organic acids, tannins, alkaloids and glycosides of cell sap in cell metabolism has not been sufficiently elucidated. Previously, they were considered as final products of exchange. It has now been shown that many of them can be re-involved in metabolic processes and therefore can be considered as reserve substances.
In addition to the function of accumulating reserve substances and waste, vacuoles in plant cells perform another important function - maintaining turgor. The concentration of ions and sugars in the cell sap of the central vacuole is usually higher than in the cell wall; The tonoplast significantly slows down the diffusion of these substances from the vacuole and at the same time is easily permeable to water. Therefore, water will enter the vacuole. This unidirectional process of water diffusion through a selectively permeable membrane is called osmosis. Water entering the cell sap exerts pressure on the wall protoplast, and through it on the cell wall, causing its tense, elastic state, or turgor cells. Turgor ensures that non-lignified plant organs retain their shape and position in space, as well as their resistance to mechanical factors.
If a cell is placed in a hypertonic solution of some non-toxic salt or sugar (i.e., in a solution of a higher concentration than the concentration of cell sap), then an osmotic release of water from the vacuole occurs. As a result of this, its volume is reduced, the elastic wall protoplast moves away from the cell wall, turgor disappears, and plasmolysis cells( Fig.2.9).
Rice. 2.9. Plasmolysis scheme: 1 – cell in a state of turgor; 2 – beginning of plasmolysis; 3 – complete plasmolysis.
Plasmolysis is usually reversible. When a cell is placed in water or a hypotonic solution, water is again vigorously absorbed by the central vacuole, the protoplast is again pressed against the cell wall, and turgor is restored. Plasmolysis can serve as an indicator of the living state of a cell; a dead cell is not plasmolyzed, since it does not have selectively permeable membranes.
Loss of turgor causes the plant to wilt. When withering in air under conditions of insufficient water supply, the thin cell walls shrink simultaneously with the protoplast and become folded.
Turgor pressure not only maintains the shape of non-lignified plant parts, it is also one of the cell growth factors, providing height cells stretching, i.e. due to the absorption of water and an increase in the size of the vacuole. Animal cells do not have a central vacuole; their growth occurs mainly due to an increase in the amount of cytoplasm, therefore the size of animal cells is usually smaller than plant cells.
The central vacuole arises from the fusion of numerous small vacuoles that are present in meristematic (embryonic) cells. These cytoplasmic vacuoles are believed to be formed by the membranes of the endoplasmic reticulum or Golgi apparatus.
Inclusions
The formation of inclusions is caused by the excessive accumulation of certain metabolic products in certain parts of the cell - in the vacuole, hyaloplasm, various organelles, and less often in the cell wall. These substances often precipitate in amorphous or crystalline form - inclusions. Inclusions have a certain shape and are clearly visible under a light microscope. By the presence of certain inclusions, their shape and distribution, one species, genera and families of plants can be distinguished from others, therefore they often serve as an important diagnostic feature in the analysis of medicinal plant materials.
Inclusions are either spare substances(compounds temporarily removed from metabolism), or final products exchange. The first category of inclusions includes starch grains,lipid drops And protein deposits; to the second - crystals some substances.
Starch grains– the most common inclusions of plant cells. Polysaccharide starch– the main type of reserve nutrients for plants. It is also the most important compound used as food by herbivores. Starch from cereal grains, potato tubers, and banana fruits is the most important source of human nutrition. Wheat flour consists of almost 75% starch grains; in potato tubers, starch makes up 20-30%. Chemically, starch is an alpha-1,4-D-glucan, the molecules have the form of branched chains, in the starch grain they are located along radii.
Starch grains are formed in the stroma of plastids. In chloroplasts, grains are deposited in light assimilative (primary) starch, formed when there is an excess of sugars - products of photosynthesis. The formation of osmotically inactive starch prevents an increase in osmotic pressure in the chloroplast. At night, when photosynthesis does not occur, assimilative starch is hydrolyzed to sugars with the help of enzymes and transported to other parts of the plant. Spare (secondary) starch deposited in amyloplasts of cells of various plant organs (roots, underground shoots, seeds) from sugars flowing from photosynthetic cells. If necessary, storage starch is also converted into sugars.
The formation of starch grains begins at certain points in the stroma of the plastid, called educational centers. Grain growth occurs by successive deposition of layers of starch around the educational center. Adjacent layers in one grain can have different refractive indexes of light, and then they are visible under a microscope - layered starch grains. The arrangement of layers can be concentric(wheat) or eccentric(potato) ( rice. 2.10). If there is one educational center in the amyloplast, around which layers of starch are deposited, then simple, if two or more, then it is formed complex grain, consisting of several simple ones. semi-compound grain is formed when starch is first deposited around several points, and then, after the contact of simple grains, common layers appear around them ( rice. 2.10).
The shape, size, number in the amyloplast and structure (position of the educational center, layering, presence or absence of cracks) of starch grains are often specific to the plant species ( rice. 2.10). Typically, starch grains are spherical, ovoid or lens-shaped, but in potatoes they are irregular. The largest grains (up to 100 microns) are characteristic of potato tuber cells; in wheat grains they are of two sizes - small (2-9 microns) and larger (30-45 microns). Corn grain cells are characterized by small grains (5-30 microns). Rice, oats, and buckwheat have complex starch grains.
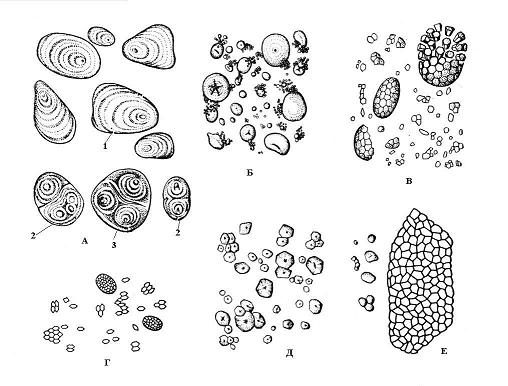
Rice. 2.10. Starch grains of various plant species: A – potatoes; B – wheat; B – oats; G – rice; D – corn; E – buckwheat; 1 – simple grain; 2 – complex grain; 3 – semi-complex grain.
The reagent for starch is a solution of iodine in a solution of potassium iodide - Lugol's reagent. It colors starch grains blue-violet.
Starch deposits are widespread in all plant organs, but seeds, underground shoots (tubers, bulbs, rhizomes), and parenchyma of the conducting tissues of the roots and stems of woody plants are especially rich in it.
Lipid drops found in almost all plant cells. Fatty oils accumulate in a huge number of plants and are the second most important form of reserve nutrients after starch. Seeds and fruits are especially rich in them. The seeds of some plants (sunflower, cotton, peanuts) can contain up to 40% oil by weight of dry matter. Therefore, vegetable fats are obtained mainly from seeds.
Lipid droplets accumulate directly in the hyaloplasm. They look like small spherical bodies, each drop is separated from the hyaloplasm by a membrane. Sometimes lipid drops are called spherosomes.
The reagent for fatty oil is a dye SudanIII, lipid droplets are colored orange-red.
Protein inclusions in the form of various amorphous or crystalline deposits are formed in various organelles of the cell. Most often, protein crystals can be found in the nucleus, less often - in the hyaloplasm, plastid stroma, in the extensions of the endoplasmic reticulum cisterns, the matrix of peroxisomes and mitochondria. The size of protein crystals is most often beyond the resolution of a light microscope.
Storage proteins belong to the category of simple proteins - proteins, unlike complex proteins - proteids, forming the basis of the protoplast. They are deposited in the greatest quantities in the storage tissue of dry seeds in the form aleurone grains, or protein bodies.
Aleurone grains are usually spherical in shape and vary in size (0.2-20 µm). They are surrounded by a membrane and contain an amorphous protein matrix, into which crystalline inclusions are immersed - one (less often, 2-3) protein crystal of rhombohedral shape and rounded globoids(from one to many) ( rice. 2.11). Globoids consist of phytin (a salt of inositol hexaphosphoric acid) and are a storage site for reserve phosphorus. Aleurone grains containing crystals are called complex. They are characteristic of storage cells of oilseeds (flax, sunflower, pumpkin, mustard, castor oil, etc.). Less common simple aleurone grains that do not contain crystals, but only amorphous protein (legumes, rice, corn) ( rice. 2.12).
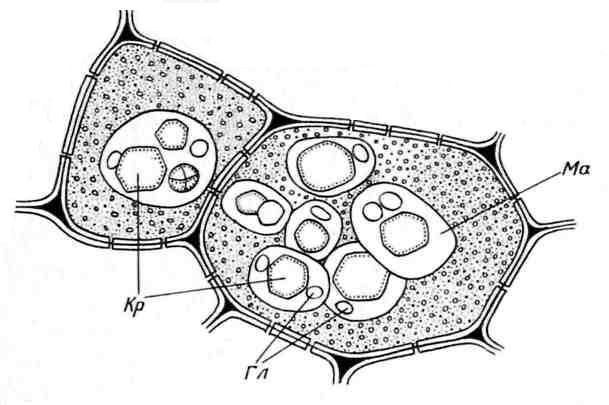
Rice. 2.11. Aleurone grains in the endosperm cells of castor bean seeds:Kr– protein crystals; Gl– globoids; Ma– protein matrix.
During seed development, storage proteins are deposited in vacuoles. When seeds ripen, accompanied by their dehydration, protein vacuoles dry out, protein and phytin precipitate out of solution and can crystallize. During seed germination, aleurone grains absorb water, swell and gradually transform into typical vacuoles. Proteins and substances of globoids are spent on the growth and development of the seedling.
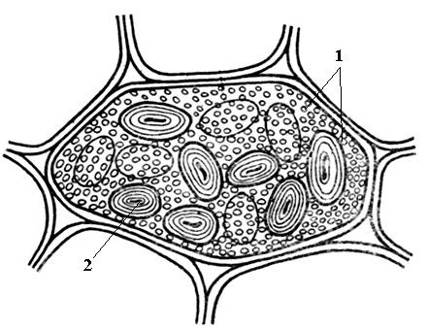
Rice. 2.12. Simple aleurone and starch grains in a bean seed cotyledon cell: 1 – simple aleurone grains; 2 – starch grain.
Protein inclusions can be stained golden yellow with Lugol's reagent.
Calcium oxalate crystals often found in plant cells. They are deposited only in vacuoles. The shape of calcium oxalate crystals is quite diverse ( rice. 2.13) and is often specific to certain plants, which is used in the diagnosis of medicinal plant materials. It can be singlecrystals rhombohedral, octahedral or elongated shape (henbane leaves), Druze– star-shaped intergrowths of spherical crystals (leaves of knotweed, datura, senna, rhubarb roots), raphids– small needle-shaped crystals collected in bunches (lily of the valley leaves, madder rhizomes), styloids– larger, rod-shaped crystals (lily of the valley leaves) and crystal sand– clusters of many small single crystals (belladonna leaves). The most common are drusen. 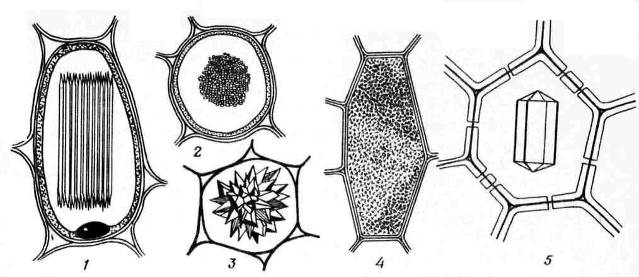
Rice. 2.13. Calcium Oxalate Crystal Forms: 1,2 – raphidas (1 – side view, 2 – cross-sectional view); 3 – drusen; 4 – crystalline sand; 5 – single crystal.
Along the fibers in the bark or along the leaf veins of a number of plants (oak bark, licorice roots, senna leaves) crystal-bearing lining– cells arranged in parallel rows with single crystals of calcium oxalate ( Fig.2.14).
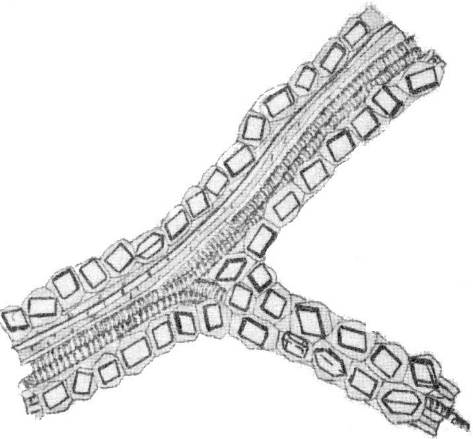
Rice. 2.14. A vein with a crystalline lining in a senna leaf.
Unlike animals, which release excess ions into the external environment along with urine, plants that do not have developed excretory organs are forced to accumulate them in their tissues. It is usually believed that calcium oxalate crystals are the end product of cell life, intended to remove excess calcium. Indeed, crystals are formed in large quantities in those organs and tissues that plants shed from time to time (leaves and bark). However, there is evidence that crystals can disappear from vacuoles. In this case, they can be considered as a place of deposition of reserve calcium.
The chemical nature of calcium oxalate crystals can be confirmed by the action of concentrated mineral acids. Under the influence of hydrochloric acid, the crystals dissolve. When exposed to sulfuric acid, calcium oxalate transforms into insoluble calcium sulfate (gypsum), forming numerous needle-shaped crystals.
Close to crystalline inclusions cystoliths. They most often consist of calcium carbonate or silica and are cluster-shaped formations that arise on the projections of the cell wall protruding into the cell ( rice. 2.15). Cystoliths are characteristic of plants of the nettle and mulberry families. The significance of cystoliths has not yet been clarified.
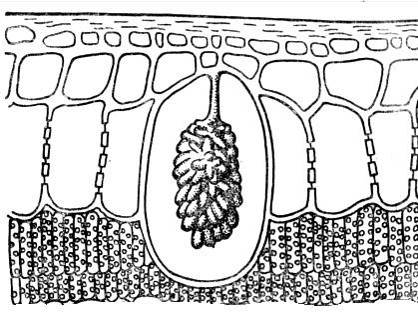
Rice. 2.15. Cystolith in the epidermal cell of a ficus leaf.
Cell wall
Cell wall (cell membrane)- a characteristic feature of a plant cell that distinguishes it from an animal cell. The cell wall gives the cell its specific shape. Plant cells cultured on special nutrient media, in which the wall is enzymatically removed, always take on a spherical shape. The cell wall gives the cell strength and protects the protoplast; it balances turgor pressure and thus prevents the rupture of the plasmalemma. The collection of cell walls forms the internal skeleton, which supports the plant body and gives it mechanical strength.
The cell wall is colorless and transparent, easily transmitting sunlight. Usually the walls are saturated with water. The cell wall system transports water and low molecular weight compounds dissolved in it (transport along the apoplast).
The cell wall consists mainly of polysaccharides, which can be divided into skeletal substances And matrix substances.
Skeletal substance plant cell wall is cellulose (fiber), which is a beta-1,4-D-glucan. This is the most common organic substance in the biosphere. Cellulose molecules are very long unbranched chains, they are located parallel to each other in groups of several dozen and are held together by numerous hydrogen bonds. As a result, microfibrils, which create the structural frame of the wall and determine its strength. Cellulose microfibrils are visible only with an electron microscope, their diameter is 10-30 nm, and their length reaches several microns.
Cellulose is insoluble and does not swell in water. It is very chemically inert and does not dissolve in organic solvents, concentrated alkalis and diluted acids. Cellulose microfibrils are elastic and very tensile (similar to steel). These properties determine the widespread use of cellulose and its products. World production of cotton fiber, which consists almost entirely of cellulose, is 1.5 10 7 tons per year. Smokeless gunpowder, acetate silk and viscose, cellophane, and paper are obtained from cellulose. A qualitative reaction for cellulose is carried out with the reagent chlorine-zinc-iodine, the cellulose cell wall turns blue-violet.
In fungi, the skeletal substance of the cell wall is chitin– a polysaccharide built from glucosamine residues. Chitin is even more durable than cellulose.
Microfibrils are immersed in amorphous matrix, usually a water-saturated plastic gel. The matrix is a complex mixture of polysaccharides, the molecules of which consist of residues of several different sugars and are shorter and branched chains than cellulose. Matrix polysaccharides determine such properties of the cell wall as strong swelling, high permeability to water and low molecular weight compounds dissolved in it, and cation exchange properties. Matrix polysaccharides are divided into two groups - pectin substances And hemicelluloses.
Pectic substances strongly swell or dissolve in water. They are easily destroyed by alkalis and acids. The simplest representatives of pectin substances are water-soluble pectic acids– polymerization products of alpha-D-galacturonic acid (up to 100 units), linked by 1,4-bonds into linear chains (alpha-1,4-D-galacturonan). Pectic acids (pectins)– these are higher molecular weight (100-200 units) polymeric compounds of alpha-D-galacturonic acid, in which the carboxyl groups are partially methylated. Pectates And pectinates– calcium and magnesium salts of pectic and pectic acids. Pectic acids, pectates and pectinates are soluble in water in the presence of sugars and organic acids to form dense gels.
The cell walls of plants contain mainly protopectins– high molecular weight polymers of methoxylated polygalacturonic acid with arabinans and galactans; in dicotyledonous plants, the galacturonan chains contain a small amount of rhamnose. Protopectins are insoluble in water.
Hemicelluloses are branched chains built from neutral sugar residues, glucose, galactose, mannose, xylose are more common; degree of polymerization 50-300. Hemicelluloses are chemically more stable than pectin substances; they are more difficult to hydrolyze and swell less easily in water. Hemicelluloses can be deposited in the cell walls of seeds as reserve substances (date palm, persimmon). Pectic substances and hemicelluloses are connected by mutual transitions. In addition to polysaccharides, a special structural protein is present in the cell wall matrix. It is bound to arabinose sugar residues and is therefore a glycoprotein.
Matrix polysaccharides do more than simply fill the spaces between cellulose microfibrils. Their chains are arranged in an orderly manner and form numerous bonds both with each other and with microfibrils, which significantly increases the strength of the cell wall.
Plant cell walls often undergo chemical modifications. Lignification, or lignification occurs when it is deposited in the matrix lignin– a polymer compound of phenolic nature, insoluble in water. The lignified cell wall loses its elasticity, its hardness and compressive strength sharply increase, and its permeability to water decreases. Reagents for lignin are: 1) phloroglucinol And concentrated hydrochloric acid or sulfuric acid(lignified walls acquire a cherry-red color) and 2) sulfataniline, under the influence of which the lignified walls become lemon-yellow. Lignification is characteristic of the cell walls of the conducting tissue of xylem (wood) and the mechanical tissue of sclerenchyma.
Sampling, or suberinization occurs as a result of deposition of hydrophobic polymers on the inside of the cell wall - suberina And wax. Suberin is a mixture of esters of polymeric fatty acids. Wax monomers are fatty alcohols and wax esters. The wax is easily extracted by organic solvents and quickly melts, forming crystals. Suberin is an amorphous compound that does not melt or dissolve in organic solvents. Suberin and wax, forming alternating parallel layers, line the entire cell cavity from the inside in the form of a film. The suberin film is practically impermeable to water and gases, so after its formation the cell usually dies. Suberization is characteristic of the cell walls of the cork's integumentary tissue. The reagent for suberized cell walls is Sudan III, orange-red color.
Coutinization The outer walls of the epidermal tissue cells are exposed. Kutin And wax deposited in alternating layers on the outer surface of the cell wall in the form of a film - cuticles. Cutin is a fat-like polymer compound similar in chemical nature and properties to suberin. The cuticle protects the plant from excessive evaporation of water from the surface of the plant. You can stain it with a reagent Sudan III in orange-red color.
Mineralization cell wall occurs due to the deposition in the matrix of a large amount of minerals, most often silica (silicon oxide), less often oxalate and calcium carbonate. Minerals give the wall hardness and brittleness. Silica deposition is characteristic of the epidermal cells of horsetails, sedges and grasses. The rigidity of stems and leaves acquired as a result of silicification serves as a protective agent against snails, and also significantly reduces the palatability and nutritional value of plants.
Some specialized cells have mucus cell wall. In this case, instead of a cellulose secondary wall, amorphous, highly hydrated acidic polysaccharides are deposited in the form slime And gums, close in chemical nature to pectin substances. Mucus dissolves well in water to form mucus solutions. Gums are sticky and stretch into threads. When dry, they have a horny consistency. When mucus is deposited, the protoplast is gradually pushed towards the center of the cell, its volume and the volume of the vacuole gradually decrease. Eventually, the cell cavity may become completely filled with mucus, and the cell dies. In some cases, mucus may pass through the primary cell wall to the surface. The Golgi apparatus plays a major role in the synthesis and secretion of mucus.
Mucus secreted by plant cells performs various functions. Thus, the mucus of the root cap serves as a lubricant, facilitating the growth of the root tip in the soil. The mucus glands of insectivorous plants (sundews) secrete trapping mucus to which insects stick. The mucilage secreted by the outer cells of the seed coat (flax, quince, plantain) secures the seed to the soil surface and protects the seedling from drying out. Mucus is stained with a reagent methylene blue in blue color.
The release of gums usually occurs when plants are wounded. For example, gum discharge from wounded areas of trunks and branches is often observed in cherries and plums. Cherry glue is a hardened gum. The gum performs a protective function, covering the wound from the surface. Gums are formed mainly in woody plants from the legume families (acacia, astragalus tragacanth) and Rosaceae of the plum subfamily (cherry, plum, apricot). Gums and mucilages are used in medicine.
The cell wall is a product of the vital activity of the protoplast. Matrix polysaccharides, wall glycoprotein, lignin and mucus are formed in the Golgi apparatus. Cellulose synthesis, formation and orientation of microfibrils are carried out by the plasmalemma. A major role in the orientation of microfibrils belongs to microtubules, which are located parallel to the deposited microfibrils near the plasmalemma. If microtubules are destroyed, only isodiametric cells are formed.
Cell wall formation begins during cell division. In the plane of division, a cell plate is formed, a single layer common to the two daughter cells. It consists of pectin substances having a semi-liquid consistency; there is no cellulose. In an adult cell, the cell plate is preserved, but undergoes changes, which is why it is called median, or intercellular plate (intercellular substance)(rice. 2.16). The median plate is usually very thin and almost indistinguishable.
Immediately after the formation of the cell plate, the protoplasts of the daughter cells begin to lay down their own cell wall. It is deposited from the inside both on the surface of the cell plate and on the surface of other cell walls that previously belonged to the mother cell. After division, the cell enters the elongation growth phase, which is caused by intense osmotic absorption of water by the cell associated with the formation and growth of the central vacuole. Turgor pressure begins to stretch the wall, but it does not tear due to the fact that new portions of microfibrils and matrix substances are constantly deposited into it. The deposition of new portions of material occurs evenly over the entire surface of the protoplast, so the thickness of the cell wall does not decrease.
The walls of dividing and growing cells are called primary. They contain a lot (60-90%) of water. The dry matter is dominated by matrix polysaccharides (60-70%), cellulose content does not exceed 30%, and there is no lignin. The thickness of the primary wall is very small (0.1-0.5 microns).
For many cells, cell wall deposition ceases simultaneously with the cessation of cell growth. Such cells are surrounded by a thin primary wall until the end of life ( rice. 2.16).
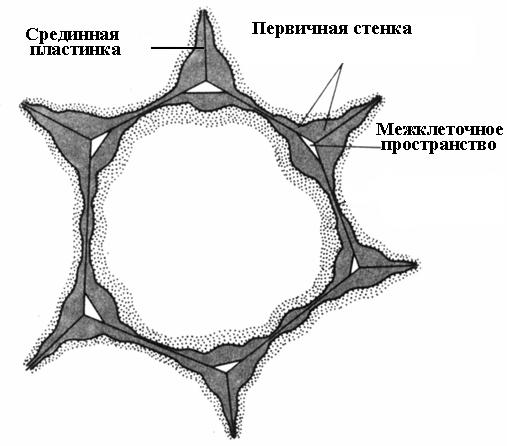
Rice. 2.16. Parenchyma cell with a primary wall.
In other cells, wall deposition continues even after the cell reaches its final size. In this case, the wall thickness increases, and the volume occupied by the cell cavity decreases. This process is called secondary thickening walls, and the wall itself is called secondary(rice. 2.17).
The secondary wall can be considered as additional, performing mainly a mechanical support function. It is the secondary wall that is responsible for the properties of wood, textile fiber, and paper. The secondary wall contains significantly less water than the primary wall; it is dominated by cellulose microfibrils (40-50% of the dry matter weight), which are located parallel to each other. Of the matrix polysaccharides, hemicelluloses (20-30%) are typical, and there are very few pectin substances. Secondary cell walls usually undergo lignification. In non-lignified secondary walls (flax bast fibers, cotton hairs), the cellulose content can reach 95%. The high content and strictly ordered orientation of microfibrils determine the high mechanical properties of the secondary walls. Often, cells that have a secondary lignified cell wall die after the secondary thickening is complete.
The median lamina glues neighboring cells together. If it is dissolved, the cell walls lose contact with each other and separate. This process is called maceration. Natural maceration is quite common, in which the pectin substances of the middle plate are converted into a soluble state using the enzyme pectinase and then washed away with water (overripe fruits of pear, melon, peach, banana). Partial maceration is often observed, in which the middle plate does not dissolve over the entire surface, but only in the corners of the cells. Due to turgor pressure, neighboring cells in these places are rounded, resulting in the formation intercellular spaces(rice. 2.16). The intercellular spaces form a single branched network, which is filled with water vapor and gases. Thus, intercellular spaces improve gas exchange of cells.
A characteristic feature of the secondary wall is its uneven deposition on top of the primary wall, as a result of which unthickened areas remain in the secondary wall - pores. If the secondary wall does not reach a large thickness, the pores look like small depressions. In cells with a strong secondary wall, the pores in cross-section look like radial channels extending from the cell cavity to the primary wall. Based on the shape of the pore channel, there are two types of pores: simple and about edged(Fig. 2.17).
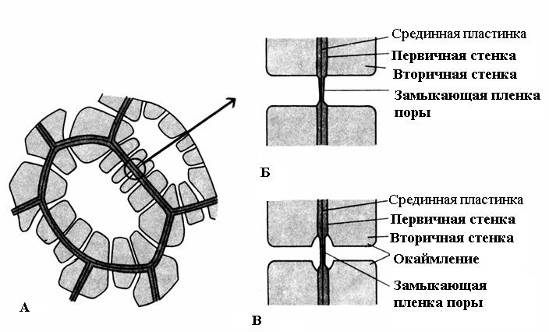
Rice. 2.17. Pore types: A – cells with secondary walls and numerous simple pores; B – a pair of simple pores; B – pair of bordered pores.
U simple pores The diameter of the pore channel is the same along its entire length and has the shape of a narrow cylinder. Simple pores are characteristic of parenchyma cells, bast and wood fibers.
Pores in two adjacent cells tend to appear opposite each other. These common pores look like one channel, separated by a thin partition of the middle plate and the primary wall. This combination of two pores of adjacent walls of neighboring cells is called pairs of pores and functions as one whole. The section of wall separating them is called closing film of the pore, or pore membrane. In living cells, the closing film of the pore is permeated with numerous plasmodesmata(rice. 2.18).
Plasmodesmata are found only in plant cells. They are strands of cytoplasm that cross the wall of adjacent cells. The number of plasmodesmata in one cell is very large - from several hundred to tens of thousands; usually plasmodesmata are collected in groups. The diameter of the plasmodesmal channel is 30-60 nm. Its walls are lined with plasmalemma, continuous with the plasmalemma of adjacent cells. In the center of the plasmodesmata there is a membrane cylinder - central rod of plasmadesmata, continuous with the membranes of the elements of the endoplasmic reticulum of both cells. Between the central rod and the plasma membrane in the canal there is hyaloplasm, continuous with the hyaloplasm of adjacent cells.
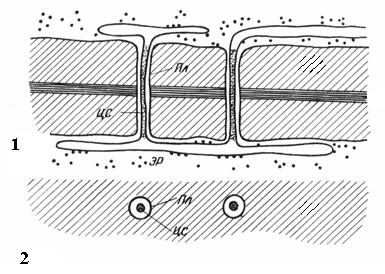
Rice. 2.18. Plasmodesmata under an electron microscope (diagram): 1 – on a longitudinal section; 2 – on a cross section; Pl– plasmalemma; CA– central rod of plasmodesmata; ER– element of the endoplasmic reticulum.
Thus, cell protoplasts are not completely isolated from each other, but communicate through plasmodesmata channels. They carry intercellular transport of ions and small molecules, and also transmit hormonal stimuli. Through plasmodesmata, protoplasts of cells in a plant organism form a single whole called simplast, and the transport of substances through plasmodesmata is called symplastic Unlike apoplastic transport along cell walls and intercellular spaces.
U bordered pores(rice. 2.17) the channel sharply narrows during the deposition of the cell wall, so the internal opening of the pore, opening into the cell cavity, is much narrower than the external one, abutting the primary wall. Bordered pores are characteristic of early dying cells of water-conducting elements of wood. In them, the pore channel expands funnel-shaped towards the closing film, and the secondary wall hangs in the form of a roller over the expanded part of the channel, forming a pore chamber. The name bordered pore comes from the fact that when viewed from the surface, the internal hole looks like a small circle or narrow slit, while the outer hole seems to border the internal one in the form of a circle of a larger diameter or a wider slit.
Pores facilitate transport into
LECTURE: Features of a plant cell
The total chemical composition is almost the same.
Similarities with animal cells: the presence of a membrane, cytosol, nucleus, and specific organelles.
However, between these types of eukaryotes there are 2 differences in structure: the presence of specific organelles and metabolism.
In a plant cell there is central vacuole. Vacuoles of plant cells absorb excess water, swell, which leads to cell stretching, the vacuole occupies most of the volume of the plant cell, and therefore only thin strands of cytoplasm remain in the plant cell, passing through the entire cell. This serves as an economical way for a plant cell to increase its size. In this case, less energy is consumed than in the case of filling the same volume with protein-rich cytoplasm.
Young plant cells may have several vacuoles, which, as the cell grows, merge with each other and form one or several large vacuoles, which occupy up to 90% of the volume of the entire cell. In this case, the organelles are pushed to the periphery of the cell, and the nucleus too. The membrane of the vacuole is the tonoplast. The vacuole cavity is filled with cell sap, which is an aqueous solution of inorganic salts, sugars, organic acids and their salts, proteins, and low molecular weight compounds.
Main function of the central vacuole– maintaining turgor pressure. It is determined by molecules dissolved in the cell sap of the vacuole that maintain osmotic concentration. The concentration ensures that the vacuole functions as a structure that maintains the internal pressure of the cell, giving it strength and tension. The tonoplast has an ATP-dependent proton pump that ensures the transport of sugars, and there are also ion channels that provide excretion (removal) from the vacuole of various metabolisms. The proton concentration in cell sap corresponds to an acidic environment of 2-5pH. The central vacuole ensures the accumulation of reserve nutrients - sugars and proteins. The supply of these substances is associated with the ability of membranes to interact with the tonoplast. Digestion processes can also take place inside the cell sap vacuole, because contain hydrolytic enzymes, while the tonoplast forms secretory vesicles, they split off from the vacuole, interact with exocetotic vesicles, ensuring the digestion of the components contained in them. They also carry out the reactions of the autophatic cycle, participating in the hydrolysis of defective filling.
Plant cell wall.
Formed with the participation of the plasmalemma. It is an extracellular multilayer formation that protects the surface of the cell, essentially being its external skeleton. It consists of two structures: a framework (made of cellulose fibril threads) and a gel-like matrix. The matrix contains polysaccharides: hemicelluloses and pectin substances. Hemicelluloses are branching polymer chains consisting of various hexoses (glucose, manose, galactose), there may also be pentoses (xylose, arabinose) and uronic acids (glucuronic and galacturonic). These components of hemicelluloses are combined with each other in different quantitative ratios and form various combinations. But the chains of hemicellulose molecules never crystallize, and their fibrils are not fixed (not detected). Contains high amounts of water.
Pectins. A heterogeneous group consisting of branched, highly hydrogenated polymers that carry negative charges due to the presence of multiple galacturonic acid residues.
Thanks to these components, the matrix is a soft plastic mass that serves as the base (frame).
Individual cellulose polymers are packaged into microfibrils using hydrogen bonds. Gives rigidity and strength. Between them there are ligaments that turn the cell wall into a monolith. Such bound cellulose microfibrils are surrounded by pectins. They can interact with calcium and silicon. What gives it rigidity. Due to the gel-like structure, the matrix provides diffuse transmission of water and small molecules.
Cell walls are divided into 3 types: primary, secondary, tertiary.
The primary cell consists of 90% carbohydrates. During cell division, a bundle of microtubules appears in the equatorial plane of the cells, located between the diverging chromosomes (phragmoplast). Among the microtubules there are many small vesicles of the vacuolar system, CG, in the central part the phragmoplasts begin to merge with each other. Forming a flattened disk or median plate. Consists of a polysaccharide called callose. More elastic compared to cellulose (not contained inside CG bubbles, formed on the plasmalemma). The CG vesicles contain the components necessary to build the membranes of two daughter cells. The process of fusion of small vacuoles occurs from the center of the cell to the periphery and continues until the membrane vesicles merge with the surface of the lateral surface of the cell.
The growing primary cell wall already consists of 3 layers (the middle plate, consisting of an amorphous matrix, and 2 peripheral ones, containing hemicellulose and fibrils). The primary wall is formed by the secretion of hemicellulose and cellulose fibrils by two new cellular structures. An increase in the thickness of the intercellular wall will occur due to the activity of daughter cells, which each, for its part, will secrete substances of the cell membrane, which thickens by layering more and more new layers. At the plasma membrane, cellulose fibrils are synthesized and polymerized, resulting in the formation of a secondary cell wall (giving the cell its final shape). As soon as the thickening of the wall is completed, it is modified with lignin, a hydrophobic polymer, synthesized through oxidative polymerization of up to three residues of aromatic alcohols and serves as the main component of wood (20-35% of wood is lignin). Due to its hydrophobic nature, lignin creates waterproofing in walls and serves as an additional strengthening material. The cell wall becomes lignified, or suberized (due to cutin and suberin). In epidermal cells, wax is secreted on the surface of the cell membranes.
Fungi have chitin (N-acetylglucosamine).
Plastids.
Common property of all plastids:
They are surrounded by two membranes that closely close along the entire surface of the organelles.
The internal contents are stroma. It contains membrane disks formed as a result of invagination and budding of sections of the inner membrane. These discs are called thylakoids and have the ability to fold into stacks (granas). They interact with the fourth type of membrane - the lamella.
Plastids have their own genome, each plastid having multiple copies of circular DNA containing about 100 genes. The genome encodes proteins needed to perform specialized functions. RNA proteins involved in transcription and translation are also located here. They originate in plastids, but most other proteins depend on the nuclear genome. These proteins are synthesized in the cytoplasm and must be imported into the plastid.
Plastids are not connected to other organelles by vesicular bonds.
Plastids represent a series of mutual transformations.
Proplastid -> leucoplast -> chloroplast -> chromoplast.
Leukoplasts can transform into: amyloplasts, elaioplasts, aleurone grains and chromoplasts.
Chloroplast.
They have an elongated shape, size 2-4 microns, and length can be 10 microns. Chromatophores (huge chloroplasts).
The internal space of the chloroplast is the lumen. 2 important phenomena: photosynthesis, energy synthesis. The thylakoid membrane is characterized by the presence of chlorophyll; it is capable of absorbing quanta of sunlight and converting solar energy into chemical energy. Absorption of light of a specific wavelength causes changes in chlorophyll. He goes into an excited state. And the released energy of activated chlorophyll is transferred through a series of intermediate stages to the electron transport chain, which leads to the synthesis of ATP and the restoration of the carrier, which is 2 mononucleotides (NAD and FAD). Energy is spent on the synthesis of carbon dioxide and the synthesis of sugars.
Photosynthesis: light and dark phase.
The light phase occurs only in the light and is associated with the absorption of light by the pigment and the conduct of a chemical reaction - the Hill reaction.
In the dark phase, carbon dioxide is fixed and reduced (from the atmosphere), which leads to the synthesis of carbon, AMK and other substances. As a result of the light phase, the process of phosphorylation occurs, the synthesis of ADP and ATP. As well as the restoration of the carrier that occurs during hydrolysis and photolysis of water.
In the light phase of photosynthesis, the energy of sunlight excites the electrons of chlorophyll. They are transported along components of the oxidative chain in the thylakoid membrane, similar to how electrons are transported along the respiratory chain in mitochondria. In chloroplasts, protons are pumped from the stroma (pH = 8) to the lumen (pH = 5) creating a gradient of 3 pH units. This proton gradient creates a proton motive force of 200 mW at the proton membrane, but this is almost entirely due to the pH gradient. Compared to the mitochondrial respiratory chain, electrons move in a different direction. Electrons are taken from water during its photolysis and transferred to the carrier with energy expenditure. That. in the light phase we have: synthesized ATP molecules and a reduced transporter. Both products are used in the dark stage.
In the dark stage, carbohydrates are formed due to reduced NAD and ATP. This process is multi-stage. A large number of enzymes are involved in it. Calvin cycle. An increase in the number of chloroplasts and the formation of other forms of plastids occurs from precursor structures (proplastids).
Proplastids are small double-membrane vesicles without distinctive features. They differ from cytoplasmic vacuoles in their denser contents and the presence of two membranes. Proplastids are located in dividing plant tissues. The number of proplastids increases through division and budding. Under normal illumination, proplastids turn into chloroplasts (increase in size, the formation of longitudinally located membrane folds occurs. Some form lamellae, others - thylakoids). If they develop in the dark, then first there is an increase in the volume of plastids, but a system of internal membranes is not formed; a mass of small vesicles is formed, which accumulate in separate zones, forming complex lattice structures - prolamellar bodies (etioplasts). Their membranes contain protochlorophyll (yellow), under the influence of light, chloroplasts are formed from them and protochlorophyll can turn into real chlorophyll (membranes, components of the transfer chain are synthesized).
Leukoplasts. Different from chloroplasts. They are found in storage tissue cells. Their uncertain morphology makes them difficult to distinguish from proplastids. They are visible as lamellae but are capable of forming normal thylakoid structures. In the dark, they accumulate various reserve substances. If it is a carbohydrate, then leukoplasts are formed into amyloplasts (there may also be proteins in the form of aleurone grains). And if fats are elaioplasts.
We observe the process of discoloration and changes in chloroplasts during the development of petals and ripening of fruits. Additional membranes accumulate in plastids, chlorophyll and starch are destroyed. When the lamellae are destroyed, lipid droplets are released, in which pigments (carotenoids) dissolve well and a change in color appears. Chromoplasts are a degenerating form of plastids, which is associated with the breakdown of lipoprotein complexes.
Plastid genome.
Its own genetic system ensures the synthesis of proteins that are localized inside the plastids themselves (thylakoid membrane proteins). They have a length of up to 60 microns. 1.3*10 8 Yes. The cycle duration and replication speed do not match. DNA has no fixing structures. All characteristics of the ring molecule of chloroplasts are close to those of prokaryotes. The similarity of chloroplast DNA is manifested in the fact that the basic regulatory processes of transcription are the same. Synthesis of all types of RNA. Chloroplast DNA codes for ribosomal RNA. 70S ribosome segmentation constants. They contain 17S and 23S. Sensitive to antibiotic (chloramphenicol).
LECTURE: Intercellular contacts
Symbiotic theory.
Komintsin and Mereshkovsky.
This theory is supported by the amazing similarity in the structure of chloroplasts and blue-green algae. Similarity with their main functions, almost identical abilities for photosynthetic processes. As a result of the symbiosis of various prokaryotic cells, nuclear (eukaryotic) cells emerged. According to this hypothesis, a eukaryotic cell in its development went through several stages of symbiosis with other cells.
In the first stage, cells such as anaerobic heterotrophic prokaryotes became hosts for aerobic bacteria, which led to the formation of aerobic prokaryotes (mitochondria, within these prokaryotic organisms). In parallel with this process, in the host cell, the prokaryotic nuclear apparatus (genophore) is formed into a nucleus isolated from the cytoplasm. This is how heterotrophic eukaryotic cells could arise. Such a cell enters into repeated symbiosis. For example, with spirochetes or flagella-like bacteria. The inclusion of these symbionts led to the appearance of flagella and cilia in heterotrophic eukaryotic cells. And as a result of additional absorption. This led to the emergence of eukaryotic animal cells with flagella. Such an organism could also absorb blue-green algae and this led to the formation of plastids and the formation of a plant cell. The hypothesis of the endosymbiotic origin of intramembrane organelles is beyond doubt. There are numerous known facts of true endosymbiosis of blue-green algae and cells of lower plants and protozoa, where the symbionts function perfectly and supply the host cell with photosynthetic products. For example. A slipper ciliate, it contains the algae chlorella. Chloroplasts can be selected by some cells and used as endosymbionts. Rotifers feed on algae. Chloroplasts find themselves inside the cells of the digestive glands and continue to perform their functions.
Features of the functions of plant cells. The method of synthesis of organic substances is autotrophic nutrition.
Plant cells, due to the presence of a cell wall, do not allow the plant cell to change its shape and move. And plant cells compensate for the lack of movement by synthesizing their own food, i.e. are autotrophs. Plants grow throughout their lives, meristems constantly divide and form new cells; all plants belong to modular organisms. Their final life form depends on their environment. Plant cells also have characteristics in the course of mitosis:
Position of the new cell wall. It is determined long before the formation of the spindle, while the spindle itself does not participate in the location of this cell. Even before mitosis, in the G 2 phase of the cell cycle, a specific structure is formed in the plant cell, which is called the preprophase ring. It is formed from microtubules of the cortex (cytoskeleton) in the form of a wide strip surrounding the cell. The nucleus remains intact (solid). In the region of this ring, actin filaments gather, which ensure its narrowing until it turns into a well-formed dense bundle of microtubules around the nucleus. The resulting structure resembles a wheel, in which the rim and spokes are composed of microtubules and actin, and the core forms the hub. The structure of the ring is enriched with EPR and KG elements. At the early stage of mitosis, the microtubules of the preprophase ring are depolymerized and destroyed. And in telophase, a new cell wall is formed exactly in the plane that was designated by the preprophase ring.
The nucleus in a plant cell moves to the center of the cell where division will occur. Narrow layers (strands) of cytoplasm extend from the nucleus in all directions. As mitosis occurs, the layers merge with each other, forming a plane where the preprophase ring used to be, and this plane is called the phragmosome.
In plants, the poles of the mitotic spindle do not contain centrioles and are more diffuse in nature. In other words, they seem to be blurred. Initially, a prophase spindle is formed from microtubules. From it, after the destruction of the nuclear membrane, a real spindle is formed, and with the formation of this real mitotic spindle, no other cytoplasmic microtubules remain in the cell. What controls this spindle, what is formed? Chromosomes. First, polymerization of microtubules occurs near the chromosomes, which are oriented randomly. Then motor proteins (dynein) sort the microtubules, their positive ends are sent to the chromosomes, which allows them to attach to the kinetochores of the chromosomes, and others interact with the negative ends of the microtubules. As a result of polymerization, sorting, and attachment, a bipolar spindle is formed. After nuclear division, cytoplasmic division occurs. This coincides with the moment of the formation of a new cell septum, the position of which is determined by the preprophase ring. The relationship between spindle orientation and new septum is not tight, as in animal cells.
Golgi complex of plant cells. Functions: glycosylation (primary), sorting (separation), synthesis of cell wall polysaccharides (except cellulose and callose), delivery of enzyme complexes (hexagonal rosettes, cellulose synthesis) to the membrane, directed transport of vesicles into vacuoles.
KG is localized in plant cells closer to the plasma, further from the nucleus, and is dispersed in the form of numerous stacks of tanks. All elements of the complex are connected by actin filaments, which form a network that ensures intracellular transport. Actin structures provide mobility (together with myosin). Plant cells have a special myosin - myosin 8. This myosin 8 does not form filaments and accumulates in newly formed primary walls; CG vesicles contain polysaccharides and structural proteins that form the secondary cell wall.
Intercellular contacts.
The contact function of membranes ensures contact of cells with each other and with others. All cells in tissues are in contact with the extracellular matrix. The extracellular matrix is involved in maintaining tissue integrity and forms an ordered framework within which cells move and interact with each other. The interaction of cells is carried out due to contacting areas, which are called intercellular contacts.
There are several classifications of them, they are structured differently. Alberts and Gray (1986) classification. According to it: contacts can be divided into 3 groups.
Mechanical (= Adhesive). Associated with the ability of cells to adhere to each other, due to the presence of extracellular matrix glycoproteins in the cell membrane. When adhesion occurs between plasma membranes, there always remains a gap (20 nm) filled with glycocalyx. Special CAM proteins (cam) are directly responsible for connecting cells to each other: fibronectin, cadherins, selectins, integrins and other proteins. Some of them connect cells to each other through intermolecular interactions. Others form special intercellular connections. The interactions between these proteins may be homophilic, i.e. neighboring cells in this case communicate with each other using homogeneous molecules. Or heterophilic, when various kinds of proteins on neighboring cells take part in adhesion. There are also more complex compounds when intercellular binding is ensured by linker intermediate molecules. In addition to such simple connections, there are a number of special structures that perform specific functions (desmosomes). They interact with elements of the cytoskeleton. There are 3 types of desmosomes: punctate, encircling and hemidesmosomes.
Dotted ones connect cells to each other in the form of buttons. The distance between the membranes of contacting cells varies from 22 to 35 nm. Between the cells, due to modifications of the supramembrane complex, a fibrous matrix is formed and in its central part there is a “plate” consisting of protein globules. They are represented by interacting integral membrane proteins cadderins and desmogleins. This plate interacts with cell membranes through a system of transverse cords. On the side of the cytoplasm, a second plate is formed from the protein desmoplakin, from which fibrillar structures related to 10 nm filaments (keratin filaments) extend in the transverse direction into the depths. Hence, punctate desmosomes are found in the epithelium, cardiac muscles, and endothelial vessels.
Girdle desmosomes. The distance between neighboring cells is 15-20 nm. The central plate is not so bright. But there are trans-membrane glycoproteins that adhere to each other and provide mechanical connection of membranes. Thin actin filaments (6-7 nm) accumulate on the cytoplasmic side. They lie along the plasmalemma and run along the entire perimeter of the cell. Function: mechanical adhesion of cells to each other; when actin filaments contract, the shape of the cell can change; can cause changes in cell geometry; transmit mechanical stress to the cell. Caused by a change in cell volume.
Hemidesmosomes. Connection of cells with the extracellular matrix (For example, with the basement membrane). The functional role is mechanical, which allows the epithelial layers to withstand large mechanical impacts. Desmosomes are parts of cells, but they are not static formations. They are dynamic. Capable of disappearing and reappearing. They are destroyed by a phagocentral reaction.
Closing (tight contacts). They are characteristic of single-layer epithelia. The zone where the outer layers of the two plasma membranes are as close as possible. Therefore, when viewed through an electron microscope, a three-layer structure is visible. The 2 outer layers of both membranes merge into one 2-3 nm thick. Not over the entire area of tight contact, but represents a series of point contacts of integral membrane proteins. More than 24 types of proteins were found in the tight junction zone. Protruding from the membranes of the bilipid layer, such proteins intersect and form a network (lattice). On the side of the tight junction, in the cytoplasm itself there are numerous fibrils (7 nm), which are located parallel to the surface of the plasmalemma. Tight junctions are characteristic of glandular and intestinal epithelia. They connect cells to each other. In addition, these are molecular structures that regulate paracellular transport, i.e. transport of substances in the space between cells. Physical barriers to ion transport are of different nature. Ions pass instantly, but other components take longer (even hours) to pass through the tight contact.
The permeability barrier in a tight contact is created by charge-carrying layers forming a network structure of fibrillar thread-like elements. Ions can pass through such pores; their size is small. However, for the passage of soluble components, the integrity of these threads must be disrupted. Soluble elements break these threads, then they are reunited after the passage of molecules. And so the molecule moves through the contact barrier. Structurally and functionally, tight junctions divide the plasmalemma into 2 sections (apical and basal) and the tight junction acts as a septum, due to which the molecular composition is maintained within limits.
Conductive contacts. It differs in animals and plants.
There are 2 types of animal cells: gap and chemical synthesis. Through gap junctions, molecules can move from one cell to another. But in chemical synthesis, cells do not have a direct connection. Gap junctions are considered to be the communication junctions of cells. This is a structure that is involved in the direct transfer of chemicals. A characteristic contact of this type is the bringing together of the plasma membranes of two neighboring cells to a distance of 2-3 nm. The gap contact itself has a size of 0.5-5 µm. It consists of 2 halves, the size of each half is 7-8 nm, they are located around a channel 1-2 nm wide. And these 2 halves are called connexons (half-channels). They consist of 6 connectin protein subunits. The molecular weight of the protein is about 30 thousand. By combining with each other, the proteins form a cylindrical structure and in two cells the size, number, size, and arrangement of connexons are strictly symmetrical. Gap contacts allow free diffusion of molecules weighing 1200 Daltons, excluding molecules whose mass reaches 2000 Daltons. The permeability of gap junctions is controlled by the opening and closing of the channel gates. This process is called gating (gate mechanism). It is controlled by changes in intracellular pH, calcium ion flux, or direct phosphorylation of connexin protein subunits. Functions of gap junctions: conduction of organic and inorganic substances. (For example, in myocardial muscle cells).
Plasmodesmata are a type of conducting contacts in plants. These are thin tubular cytoplasmic channels connecting 2 neighboring cells. The diameter of these channels varies from 20 to 40 nm. The peculiarity of these channels is that the plasma membrane of one cell passes into the plasma membrane of another cell. Along the axis of this channel, a cylindrical tube of the desmotule stretches from one cell to another. Its lumen communicates with the ER cells. The space between the desmotubule and the plasmodesmata membrane is filled with cytosol. The plasmalemma that borders these cells smoothly passes into the plasmodesmata and the membrane of other cells. As a result, the hyaloplasm of neighboring cells is connected into a single system (symplast). And formally there is no separation (one cell from another). Free transport through plasmodesmata is limited to a molecular weight of 800 Daltons. Plasmodesmata are formed at the stage of the primary formation of the median plate from the elements of the ER. In a newly divided cell, the number of plasmodesmata reaches up to 1000 per cell. With aging, their number decreases, but their thickness increases. Functional role: ensuring intercellular circulation of solutions (ions, sugars, nutrients). The negative side is that it is easier to get sick.
