7.11. The structure of substances with covalent bonds
Substances in which, of all types of chemical bonds, only a covalent one is present, are divided into two unequal groups: molecular (very many) and non-molecular (much less).
Crystals of solid molecular substances consist of molecules weakly bound together by the forces of intermolecular interaction of molecules. Such crystals do not have high strength and hardness (think ice or sugar). Their melting and boiling points are also low (see Table 22).
Table 22. Melting and boiling points of some molecular substances
Substance |
Substance |
||||
| H 2 | – 259 | – 253 | BR 2 | – 7 | 58 |
| N 2 | – 210 | – 196 | H2O | 0 | 100 |
| HCl | – 112 | – 85 | P 4 | 44 | 257 |
| NH 3 | – 78 | – 33 | C 10 H 8 (naphthalene) | 80 | 218 |
| SO 2 | – 75 | – 10 | S 8 | 119 |
Unlike their molecular counterparts, non-molecular substances with covalent bonds form very hard crystals. Diamond crystals (the hardest substance) belong to this type.
In a diamond crystal (Fig. 7.5), each carbon atom is connected to four other carbon atoms by simple covalent bonds (sp 3 hybridization). The carbon atoms form a three-dimensional framework. Essentially the entire diamond crystal is one huge and very strong molecule.
Silicon crystals, widely used in radio electronics and electronic engineering, have the same structure.
If you replace half of the carbon atoms in diamond with silicon atoms without disturbing the framework structure of the crystal, you will get a crystal of silicon carbide SiC - also a very hard substance used as an abrasive material. Ordinary quartz sand (silicon dioxide) also belongs to this type of crystalline substance. Quartz is a very hard substance; Under the name "emery" it is also used as an abrasive material. The quartz structure is easily obtained by inserting oxygen atoms between every two silicon atoms in a silicon crystal. In this case, each silicon atom will be associated with four oxygen atoms, and each oxygen atom with two silicon atoms.
Crystals of diamond, silicon, quartz and similar structures are called atomic crystals.
An atomic crystal is a crystal consisting of atoms of one or more elements linked by chemical bonds.
A chemical bond in an atomic crystal can be covalent or metallic.
As you already know, any atomic crystal, like an ionic crystal, is a huge “supermolecule”. The structural formula of such a “supermolecule” cannot be written down - you can only show its fragment, for example:

Unlike molecular substances, substances that form atomic crystals are among the most refractory (see table 23.).
Table 23. Melting and boiling points of some non-molecular substances With covalent bonds
Such high melting temperatures are quite understandable if we remember that when these substances melt, it is not weak intermolecular bonds that are broken, but strong chemical bonds. For the same reason, many substances that form atomic crystals do not melt when heated, but decompose or immediately transform into a vapor state (sublimate), for example, graphite sublimes at 3700 o C.
| Silicon – Si. Very hard, brittle silicon crystals look like metal, but it is nevertheless a non-metal. Based on the type of electrical conductivity, this substance is classified as a semiconductor, which determines its enormous importance in the modern world. Silicon is the most important semiconductor material. Radios, televisions, computers, modern telephones, electronic watches, solar panels and many other household and industrial devices contain transistors, microcircuits and photocells made from single crystals of high-purity silicon as the most important structural elements. Technical silicon is used in steel production and non-ferrous metallurgy. In terms of its chemical properties, silicon is a fairly inert substance; it reacts only at high temperatures. Silicon dioxide – SiO 2 . Another name for this substance is silica. Silicon dioxide occurs in nature in two forms: crystalline and amorphous. Many semi-precious and ornamental stones are varieties of crystalline silicon dioxide (quartz): rock crystal, jasper, chalcedony, agate. and opal is an amorphous form of silica. Quartz is very widespread in nature, because dunes in deserts and sandbanks of rivers and seas are all quartz sand. Quartz is a colorless crystalline, very hard and refractory substance. It is inferior in hardness to diamond and corundum, but, nevertheless, is widely used as an abrasive material. Quartz sand is widely used in construction and the building materials industry. Quartz glass is used to make laboratory glassware and scientific instruments because it does not crack under sudden changes in temperature. In terms of its chemical properties, silicon dioxide is an acidic oxide, but it reacts with alkalis only when fused. At high temperatures, silicon dioxide and graphite are used to produce silicon carbide - carborundum. Carborundum is the second hardest substance after diamond; it is also used to make grinding wheels and “sandpaper”. |
7.12. Polarity of a covalent bond. Electronegativity
Recall that isolated atoms of different elements have different propensities to both give up and accept electrons. These differences persist after the formation of a covalent bond. That is, atoms of some elements tend to attract the electron pair of a covalent bond to themselves more strongly than atoms of other elements.
Consider a molecule HCl.
Using this example, let's see how we can estimate the displacement of the electron communication cloud using molar ionization energies and means to the electron. 1312 kJ/mol, and 1251 kJ/mol - the difference is insignificant, approximately 5%. 73 kJ/mol, and 349 kJ/mol - here the difference is much greater: the electron affinity energy of the chlorine atom is almost five times greater than that for the hydrogen atom. From this we can conclude that the electron pair of the covalent bond in the hydrogen chloride molecule is largely shifted towards the chlorine atom. In other words, the bonding electrons spend more time near the chlorine atom than near the hydrogen atom. This uneven distribution of electron density leads to a redistribution of electrical charges inside the molecule. Partial (excess) charges arise on the atoms; on the hydrogen atom it is positive, and on the chlorine atom it is negative.
In this case, the bond is said to be polarized, and the bond itself is called a polar covalent bond.
If the electron pair of a covalent bond is not displaced to any of the bonded atoms, that is, the bond electrons equally belong to the bonded atoms, then such a bond is called a nonpolar covalent bond.
The concept of "formal charge" in the case of a covalent bond is also applicable. Only in the definition we should not be talking about ions, but about atoms. In general, the following definition can be given.
In molecules in which covalent bonds are formed only by an exchange mechanism, the formal charges of the atoms are equal to zero. Thus, in the HCl molecule, the formal charges on both the chlorine and hydrogen atoms are zero. Consequently, in this molecule the real (effective) charges on the chlorine and hydrogen atoms are equal to the partial (excess) charges.
It is not always easy to determine the sign of the partial charge on an atom of one or another element in a molecule based on the molar ionization energies and affinity for the electrode, that is, to estimate in which direction the electron pairs of bonds are shifted. Usually, for these purposes, another energy characteristic of an atom is used - electronegativity.
Currently, there is no single, generally accepted designation for electronegativity. It can be denoted by the letters E/O. There is also no single, generally accepted method for calculating electronegativity. In a simplified way, it can be represented as half the sum of the molar ionization energies and electron affinity - this was one of the first ways to calculate it.
The absolute values of electronegativity of atoms of various elements are used very rarely. The most commonly used is relative electronegativity, denoted by c. Initially, this value was defined as the ratio of the electronegativity of an atom of a given element to the electronegativity of a lithium atom. Subsequently, the methods of its calculation changed somewhat.
Relative electronegativity is a dimensionless quantity. Its values are given in Appendix 10.
Since relative electronegativity depends primarily on the ionization energy of the atom (electron affinity energy is always much lower), then in a system of chemical elements it changes approximately the same as the ionization energy, that is, it increases diagonally from cesium (0.86) to fluorine (4.10). The values of the relative electronegativity of helium and neon given in the table have no practical significance, since these elements do not form compounds.
Using the electronegativity table, you can easily determine towards which of the two atoms the electrons connecting these atoms are shifted, and, therefore, the signs of the partial charges arising on these atoms.
| H2O | The connection is polar | |||
| H 2 | Atoms are the same | H--H | The connection is non-polar | |
| CO2 | The connection is polar | |||
| Cl2 | Atoms are the same | Cl--Cl | The connection is non-polar | |
| H2S | The connection is polar |
Thus, in the case of the formation of a covalent bond between atoms of different elements, such a bond will always be polar, and in the case of the formation of a covalent bond between atoms of the same element (in simple substances), the bond is in most cases non-polar.
The greater the difference in electronegativity of the bonded atoms, the more polar the covalent bond between these atoms turns out to be.
| Hydrogen sulfide H 2 S– a colorless gas with a characteristic odor characteristic of rotten eggs; poisonous. It is thermally unstable and decomposes when heated. Hydrogen sulfide is slightly soluble in water; its aqueous solution is called hydrosulfide acid. Hydrogen sulfide provokes (catalyzes) corrosion of metals; it is this gas that is “to blame” for the darkening of silver. It is naturally found in some mineral waters. In the process of life, it is formed by some bacteria. Hydrogen sulfide is destructive to all living things. A hydrogen sulfide layer was discovered in the depths of the Black Sea and causes concern to scientists: the life of marine inhabitants there is under constant threat. |
POLAR COVALENT BOND, NON-POLAR COVALENT BOND, ABSOLUTE ELECTRONEGATIVITY, RELATIVE ELECTRONEGATIVITY.
1. Experiments and subsequent calculations showed that the effective charge of silicon in silicon tetrafluoride is +1.64 e, and of xenon in xenon hexafluoride +2.3 e. Determine the values of the partial charges on the fluorine atoms in these compounds. 2. Make up the structural formulas of the following substances and, using the notations " " and " ", characterize the polarity of covalent bonds in the molecules of these compounds: a) CH 4, CCl 4, SiCl 4; b) H 2 O, H 2 S, H 2 Se, H 2 Te; c) NH 3, NF 3, NCl 3; d) SO 2, Cl 2 O, OF 2.
3.Using the electronegativity table, indicate in which of the compounds the bond is more polar: a) CCl 4 or SiCl 4 ; b) H 2 S or H 2 O; c) NF 3 or NCl 3; d) Cl 2 O or OF 2.
7.13. Donor-acceptor mechanism of bond formation
In the previous paragraphs, you learned in detail about two types of bonds: ionic and covalent. Recall that an ionic bond is formed when an electron is completely transferred from one atom to another. Covalent - when sharing unpaired electrons of bonded atoms.
In addition, there is another mechanism for bond formation. Let's consider it using the example of the interaction of an ammonia molecule with a boron trifluoride molecule:

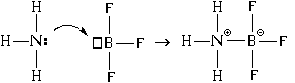
As a result, both covalent and ionic bonds arise between the nitrogen and boron atoms. In this case, the nitrogen atom is donor electron pair ("gives" it for the formation of a bond), and the boron atom - acceptor(“accepts” it when forming a connection). Hence the name of the mechanism for the formation of such a connection - “ donor-acceptor".
When a bond is formed using the donor-acceptor mechanism, both a covalent bond and an ionic bond are formed simultaneously.
Of course, after the formation of a bond, due to the difference in the electronegativity of the bonded atoms, polarization of the bond occurs and partial charges arise, reducing the effective (real) charges of the atoms.
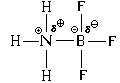
Let's look at other examples.
If there is a highly polar hydrogen chloride molecule next to the ammonia molecule, in which there is a significant partial charge on the hydrogen atom, then in this case the role of the electron pair acceptor will be played by the hydrogen atom. Its 1 s-AO, although not completely empty, like the boron atom in the previous example, the electron density in the cloud of this orbital is significantly reduced.

The spatial structure of the resulting cation is ammonium ion NH 4 is similar to the structure of the methane molecule, that is, all four N-H bonds are exactly the same.
The formation of ionic crystals of ammonium chloride NH 4 Cl can be observed by mixing ammonia gas with hydrogen chloride gas:
NH 3 (g) + HCl (g) = NH 4 Cl (cr)
Not only the nitrogen atom can be an electron pair donor. It could be, for example, the oxygen atom of a water molecule. A water molecule will interact with the same hydrogen chloride as follows:
The resulting H3O cation is called oxonium ion and, as you will soon learn, is of great importance in chemistry.
In conclusion, let us consider the electronic structure of the carbon monoxide (carbon monoxide) CO molecule:
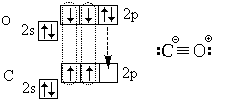
In addition to three covalent bonds (triple bond), it also contains an ionic bond.
Conditions for bond formation according to the donor-acceptor mechanism:
1) the presence of a lone pair of valence electrons in one of the atoms;
2) the presence of a free orbital on the valence sublevel of another atom.
The donor-acceptor mechanism of bond formation is quite widespread. It occurs especially often during the formation of compounds d-elements. Almost everyone's atoms d-elements have many empty valence orbitals. Therefore, they are active acceptors of electron pairs.
DONOR-ACCEPTOR MECHANISM OF BOND FORMATION, AMMONIUM ION, OXONIUM ION, CONDITIONS FOR BOND FORMATION BY DONOR-ACCEPTOR MECHANISM.
1.Make reaction equations and formation schemes
a) ammonium bromide NH 4 Br from ammonia and hydrogen bromide;
b) ammonium sulfate (NH 4) 2 SO 4 from ammonia and sulfuric acid.
2. Create reaction equations and interaction schemes for a) water with hydrogen bromide; b) water with sulfuric acid.
3.Which atoms in the four previous reactions are donors of an electron pair, and which are acceptors? Why? Explain your answer with diagrams of valence sublevels.
4.Structural formula of nitric acid. The angles between O–N–O bonds are close to 120 o. Define:
a) type of hybridization of the nitrogen atom;
b) which AO of the nitrogen atom takes part in the formation of the -bond;
c) which AO of the nitrogen atom takes part in the formation of an -bond according to the donor-acceptor mechanism.
What do you think the angle between the H–O–N bonds in this molecule is approximately equal to? 5.Create the structural formula of the cyanide ion CN (negative charge on the carbon atom). It is known that cyanides (compounds containing such an ion) and carbon monoxide CO are strong poisons, and their biological effect is very similar. Offer your explanation of the proximity of their biological action.
7.14. Metal connection. Metals
A covalent bond is formed between atoms that are similar in their propensity to give up and gain electrons only when the sizes of the bonded atoms are small. In this case, the electron density in the region of overlapping electron clouds is significant, and the atoms turn out to be tightly bound, as, for example, in the HF molecule. If at least one of the bonded atoms has a large radius, the formation of a covalent bond becomes less advantageous, since the electron density in the region of overlapping electron clouds for large atoms is much less than for small ones. An example of such a molecule with a weaker bond is the HI molecule (using Table 21, compare the atomization energies of HF and HI molecules).
And yet between large atoms ( r o > 1.1) a chemical bond occurs, but in this case it is formed due to the sharing of all (or part) of the valence electrons of all bonded atoms. For example, in the case of sodium atoms, all 3 s-electrons of these atoms, and a single electron cloud is formed:
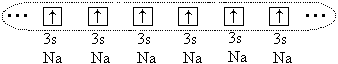
Atoms form a crystal with metal communication
In this way, both atoms of the same element and atoms of different elements can bond with each other. In the first case, simple substances called metals, and in the second - complex substances called intermetallic compounds.
Of all the substances with metallic bonds between atoms, you will only learn about metals in school. What is the spatial structure of metals? The metal crystal consists of atomic skeletons, remaining after the socialization of valence electrons, and the electron cloud of socialized electrons. The atomic cores usually form a very close packing, and the electron cloud occupies the entire remaining free volume of the crystal.
The main types of dense packaging are cubic closest packing(KPU) and hexagonal close packing(GPU). The names of these packages are associated with the symmetry of the crystals in which they are realized. Some metals form loosely packed crystals - body-centered cubic(OTSK). Volume and ball-and-stick models of these packages are shown in Figure 7.6.
Cubic close packing is formed by atoms of Cu, Al, Pb, Au and some other elements. Hexagonal close packing - atoms of Be, Zn, Cd, Sc and a number of others. Body-centered cubic packing of atoms is present in crystals of alkali metals, elements of VB and VIB groups. Some metals may have different structures at different temperatures. The reasons for such differences and structural features of metals are still not fully understood.
When melted, metal crystals turn into metal liquids. The type of chemical bond between atoms does not change.
The metal bond does not have directionality and saturation. In this respect it is similar to an ionic bond.
In the case of intermetallic compounds, we can also talk about the polarizability of the metallic bond.
Characteristic physical properties of metals:
1) high electrical conductivity;
2) high thermal conductivity;
3) high ductility.

The melting points of different metals are very different from each other: the lowest melting point is for mercury (- 39 o C), and the highest is for tungsten (3410 o C).
| Beryllium Be- light gray, lightweight, fairly hard, but usually brittle metal. Melting point 1287 o C. In air it becomes covered with an oxide film. Beryllium is a fairly rare metal; living organisms in the process of their evolution had practically no contact with it, so it is not surprising that it is poisonous to the animal world. It is used in nuclear technology. Zinc Zn is a white soft metal with a bluish tint. Melting point 420 o C. In air and water it is covered with a thin dense film of zinc oxide, which prevents further oxidation. In production it is used for galvanizing sheets, pipes, wires, protecting iron from corrosion. Wolfram W. It is the most refractory of all metals: the melting point of tungsten is 3387 o C. Typically, tungsten is quite brittle, but after careful cleaning it becomes ductile, which makes it possible to draw thin wire from it, from which the filaments of light bulbs are made. However, most of the tungsten produced is used for the production of hard and wear-resistant alloys that can retain these properties when heated even to 1000 o C. |
METAL, INTERMETALLIC COMPOUND, METALLIC BOND, DENSE PACKING.
1. To characterize various packages, the concept of “space filling coefficient” is used, that is, the ratio of the volume of atoms to the volume of the crystal
Where V a - volume of an atom,
Z is the number of atoms in a unit cell,
V i- volume of the unit cell.
Atoms in this case are represented by rigid balls of radius R, touching each other. Ball volume V w = (4/3) R 3 .
Determine the space filling factor for bulk and bcc packaging.
2. Using the values of metal radii (Appendix 9), calculate the unit cell size of a) copper (CPU), b) aluminum (CPU) and c) cesium (BCC).
Vanderwaals forces
Van der Waals forces (Van der Waals, Dutch scientist, 1873) determine the interaction between molecules. They include
dipole-dipole, induction and dispersion interactions.
Dipole-dipole interaction occurs between opposite poles of permanent dipoles. Induction interactions occur between dipoles and non-polar molecules. A dipole acts on a nonpolar molecule turning it into an induced dipole. An attraction occurs between the permanent and induced dipoles, the energy of which is proportional to the distance between the centers of the molecules. The energy of inductive interaction increases with increasing polarizability of molecules (the ability of a molecule to form a dipole).
Dispersive interaction occurs between instantaneous dipoles of nonpolar molecules. Electrical density fluctuations occur in any molecule, resulting in the appearance of instantaneous dipoles, which in turn induce instantaneous dipoles in neighboring molecules.
The energy of all types of interactions considered is inversely proportional to the distance between the centers of the molecules to the sixth power.
3.2. Hydrogen bond
A hydrogen bond occurs as a result of the interaction of positively polarized hydrogen of one molecule and a negatively polarized atom of another molecule. For example, ...H + ─F - ...H + ─F - ... If a hydrogen bond is formed inside a molecule, it is called intramolecular.
When hydrogen bonds occur, dimers, trimmers, or polymer structures are formed. This leads to an increase in viscosity, dielectric constant, boiling and melting points, heats of fusion and vaporization.
Spatial configuration of molecules
The spatial structure of molecules is determined by the number of atoms in the molecule and the direction of chemical bonds.
Diatomic molecules (H 2), triatomic molecules CaCl 2, C 2 H 2) have a linear structure.
Triatomic molecules can have an angular structure (H 2 S, H 2 O); pyramidal (NH 3); flat triangle (AlCl 3, BF 3).
Polyatomic molecules have more complex configurations - tetrahedral (CH 4), octahedral (SF 6); cyclic (C 4 H 8, C 6 H 6), etc.
Hydrogen fluoride
hydrogen sulfide
Boron fluoride
Test tasks
1. In which molecules is the chemical bond polar? F2, CO, N2, HBr?
2. Determine the valency of fluorine and phosphorus in the ground and excited states.
3. Indicate the mechanism of formation of chemical bonds in molecules of CO, water, ammonium hydroxide, ammonium cation.
4. What proazo, magnesium chloride, aluminum bromide, hydrogen selenide, acetylene, ppene, pentane?
5. Give the electronic configurations of oxygen and hydrogen chloride molecules using the MO method.
6. Using the MS method, determine whether the formation of ions is possible
H 2 + , He 2 + , O 2 - .
7. Draw energy diagrams of nitrogen and fluorine molecules using the MO method.
8. What types of van der Waals forces are present in the molecules of hydrogen peroxide, water, hydrogen bromide, ethyl alcohol, methanal?
9. What parameters does the length of a chemical bond depend on?
10. How does the energy of a chemical bond change in the hydrogen halide series from hydrogen fluoride to hydrogen iodide?
11. How does the energy of the chemical bond between carbons change in the series: single, double, triple, aromatic?
Formation and properties of chemical bonds
A chemical bond is formed only if, as atoms approach each other, the total energy of the system decreases.
Chemical bonding occurs when clouds of unpaired electrons with antiparallel spins overlap due to the electrostatic interaction of atomic nuclei with increased electron density between them.
Let us consider, for example, the formation of a bond in a hydrogen molecule. When hydrogen atoms come together, their electron clouds penetrate each other, which is called electron cloud overlap (Fig. 4.1).
Rice. 4.1.Overlapping of electron clouds during the formation of a hydrogen molecule.
The electron density between nuclei increases. The nuclei attract each other. As a result, the energy of the system decreases (Fig. 4.2). However, when the atoms are very close together, the repulsion of nuclei increases sharply. Optimal distance between cores – communication length (l sv), at which the system has minimal energy. When atoms transition to this state, energy is released, called binding energy (E St).
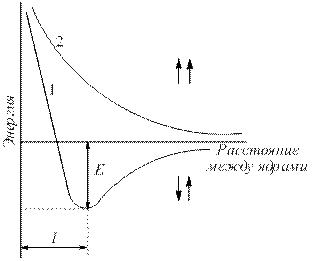
Rice. 4.2 .
Dependence of the energy of systems of two hydrogen atoms with parallel (1) and antiparallel (2) spins on the distance between the nuclei (E – binding energy).
If the bond is formed by identical atoms, for example H–H, Cl–Cl, NºN, then the shared electrons are evenly distributed between them. This connection is called covalent nonpolar communication.
If one of the atoms attracts electrons more strongly, then the electron pair shifts towards this atom. In this case, there is polar covalent connection. Electronegativity (EO) can serve as a criterion for the ability of an atom to attract an electron. The higher the EO of an atom, the more likely it is that an electron pair will shift toward the nucleus of that atom. Therefore, the difference in electronegativity of atoms characterizes communication polarity.
In the case of a large difference in the EO of atoms, for example, between s-metals of the first and second groups of the periodic system and non-metals of groups VI, VII (LiF, CsCl, K 2 O, etc.), not only a shift in the electron density, but also a complete transition can occur electron to a more electronegative atom to form a positive and a negative ion. Ionic chemical bond is the electrostatic interaction of negatively and positively charged ions in a chemical compound.
Thus, the bonds differ in polarity.
Communication polarity
Communication polarity is due to the different electronegativity of the atoms between which a chemical bond is formed.
Due to the displacement of the electron bond pair to a more electronegative atom, it acquires a partial (effective) negative charge (δ–). The second atom acquires a partial positive charge (δ+). This is how a dipole arises, which is an electrically neutral system with two equal-sized positive and negative charges located at a certain distance l d from each other (dipole length). A measure of bond polarity is the electric moment of the dipole (μm), equal to the product of the effective charge and the length of the dipole l d.
| . | (4.1) |
The electric moment of a dipole has the unit of measurement coulomb per meter (C×m). The non-system unit of measurement debye – D, equal to 3.3 × 10 –30 C × m (Table 4.1) is also used as a unit of measurement.
As can be seen from table. 4.1, the electric dipole moment increases with increasing EO difference.
Table 4.1
Electric dipole moment of a chemical bond in molecules
| Connection | EO difference | μ St, D | Connection | EO difference | μ St, D |
| H–F | 1,9 | 1,98 | BUT | 1,4 | 1,53 |
| Н–Сl | 0,9 | 1,03 | N–N | 0,9 | 1,3 |
| H–Br | 0,74 | 0,78 | H–S | 0,4 | 0,7 |
| Н–I | 0,4 | 0,38 | N–S | 0,4 | 0,3 |
A polar covalent bond with δ approaching 1 can be considered an ionic bond. However, even for ionic compounds δ is below unity. Therefore, any ionic bond has a certain amount of covalency. The chemical bond in most chemical compounds combines the properties of covalent and ionic bonds. The polarity of the connection may change.
During the interaction of atoms whose electronegativity values differ, but not sharply, the common electron pair shifts to a more electronegative atom. This is the most common type of chemical bond, found in both inorganic and organic compounds. Covalent bonds fully include those bonds that are formed according to the donor-acceptor mechanism. formation of ammonium ion by a donor-acceptor mechanism.
9 Valence bond method.
The valence bond method was first used in 1927 by German scientists W. Heitler and F. London, who carried out a quantum mechanical calculation of the hydrogen atom. The BC method assumes that the atoms in a molecule retain their individuality. An electron pair populates the orbital of one or the other atom. Heitler and London showed that when two hydrogen atoms with antiparallel spins approach each other, the energy of the system decreases, which is due to an increase in the electron density in the space between the nuclei of the interacting atoms. When atoms with parallel spins come closer together, the energy of the system increases and in this case a molecule is not formed. The BC method is based on the following basic principles: 1) a chemical bond between two atoms arises as a result of the overlapping of AOs with the formation of electron pairs. 2) atoms entering into a chemical bond exchange electrons with each other, which form bonding pairs. Only unpaired electrons of atoms can participate in the formation of common electron pairs. The energy of electron exchange between atoms makes the main contribution to the energy of a chemical bond. An additional contribution is made by the Coulomb forces of particle interaction. 3) in accordance with the Pauli principle, a chemical bond is formed only when electrons with antiparallel spins interact. 4) the characteristics of the chemical bond are determined by the type of AO overlap
Curve of binding energy between molecules versus distance
10 Valence – ability of an atom to form chemical bonds. A measure of valence is the number of chemical bonds. The valence capabilities of an atom are determined by the number of unpaired (valence) electrons on the outer layer and the number of bonds that can be formed by the donor-acceptor mechanism. Oxidation state - the conventional charge of an atom in a molecule, calculated under the assumption that all bonds are ionic in nature. This means that a more electronegative atom, displacing one electron pair toward itself, acquires a charge of -1, and two electron pairs acquire a charge of -2. The bond between identical atoms does not contribute to the oxidation state. Thus, the bond between C-C atoms corresponds to their zero oxidation state. In the C-H bond, carbon, as a more electronegative atom, has a charge of -1, and in a C-O bond, the charge of carbon (less electronegative) is +1. The oxidation state of an atom in a molecule is calculated as the algebraic sum of the charges that all bonds of a given atom give. Thus, in the CH 3 Cl molecule, three C-H bonds give a total charge on the C atom equal to -3, and the C-Cl bond gives a charge of +1. Therefore, the oxidation state of the carbon atom in this compound is:
In an excited state, the valence of atoms increases. This is due to the phenomenon of steaming and promotion( transition to a free orbital) of electrons in the outer layer.
11 .
Chemical bond- interatomic interaction caused by the overlap of the outer electron shells of atoms, accompanied by a decrease in the total energy of the resulting system. A chemical bond can be formed by donating one or more unpaired electrons from each atom (multiple bonds) to form electron pairs (covalent bond), or by one atom dominating an electron pair and the other atom occupying a vacant electron orbital (donor-acceptor bond). Only electrons from the outer electron shell participate in the formation of a chemical bond, and the internal electron levels are not affected. As a result, when a chemical bond is formed, each atom forms a filled electron shell of the outer electronic level, consisting of two (doublet) or eight (octet) electrons. A chemical bond is characterized by length and energy. The length of a chemical bond is the distance between the nuclei of bonded atoms. The energy of a chemical bond shows how much energy must be expended to separate two atoms between which a chemical bond exists to the distance at which this chemical bond will be broken. Main types of chemical bond- covalent, ionic, hydrogen, metallic.
Communication saturation provides a constant composition of molecules and defines the concept of valence. If an atom has n unpaired electrons, then this atom can form n chemical bonds with other atoms that each have one unpaired electron. Therefore, the valency of an element is equal to the number of unpaired electrons in the atom or the number of covalent bonds formed. The saturation principle is derived from the Pauli principle and means that each electron can participate in the formation of only one covalent bond. Polarity of chemical bonds - characteristic of a chemical bond, showing a change in the distribution of electron density in the space around the nuclei in comparison with the distribution of electron density in the neutral atoms forming this bond.
The so-called effective charges on atoms are used as a quantitative measure of bond polarity.
The effective charge is defined as the difference between the charge of electrons located in some region of space near the nucleus and the charge of the nucleus. However, this measure has only a conditional and approximate meaning, since it is impossible to unambiguously identify a region in a molecule that relates exclusively to an individual atom, and in the case of several bonds, to a specific bond.
The presence of an effective charge can be indicated by symbols of charges on atoms (for example, H + δ - Cl − δ, where δ is a certain fraction of the elementary charge).
12 Hydrogen bond. This type of bond can only conditionally be called chemical and is more correctly attributed to intermolecular and intramolecular interactions. A hydrogen bond occurs between a bonded hydrogen atom of one molecule and an electronegative atom of another molecule. The hydrogen bond is partly electrostatic and partly donor-acceptor in nature. A clear example of the implementation of such a connection can be the combination of several water molecules into clusters. In a water molecule, the oxygen atom shifts its electron density to itself, acquiring a partial negative charge, and the hydrogen, accordingly, is partially positive and can interact with the lone electron pair of oxygen of the neighboring molecule. A hydrogen bond can occur not only between different molecules, but also within the molecule itself. Van der Waals interaction occurs due to the occurrence of induced dipole moments. This type of interaction can occur both between different molecules and within one molecule between neighboring atoms due to the appearance of a dipole moment in the atoms during the movement of electrons. Van der Waals interaction can be attractive or repulsive. Intermolecular interaction is of the nature of attraction, and intramolecular interaction is of repulsion. Intramolecular van der Waals interactions have a significant contribution to the geometry of the molecule. The formation of intermolecular hydrogen bonds leads to a significant change in the properties of substances: an increase in viscosity, dielectric constant, melting and boiling points, heats of vaporization and melting. For example, water , hydrogen fluoride and ammonia have abnormally high boiling and melting points. Under the influence of hydrogen bonds, chemical properties also change. Since many compounds contain covalent polar H–O and H–N bonds, hydrogen bonds are very common. They appear not only in water, but also in various crystalline substances, polymers, proteins, living organisms... Due to their low values energy hydrogen bonds are relatively easily broken and re-formed. The energy of a hydrogen bond increases with increasing electronegativity (EO) and decreasing sizes of B atoms. Therefore, the strongest hydrogen bonds occur when the B atoms are F, O or N
13-14 Depending on the distance between the particles that make up the substance and on the nature and energy intermolecular interaction (IMI) between them, a substance can be in one of three states of aggregation: solid, liquid and gaseous.
IN gas state, the energy of interaction between particles is much less than their kinetic energy:
E MMV<< Е кин .
Therefore, gas molecules (atoms) are not held together, but move freely in a volume significantly larger than the volume of the particles themselves. Intermolecular interaction forces appear when molecules come close enough to each other. Weak intermolecular interaction determines the low density of the gas, the desire for limitless expansion, and the ability to exert pressure on the walls of the vessel, which impede this desire. Gas molecules are in random, chaotic motion, and there is no order in the gas regarding the arrangement of molecules.
The state of a gas is characterized by: temperature - T, pressure - p and volume - V. At low pressures and high temperatures, all typical gases behave approximately the same. But already at ordinary and, especially, low temperatures and high pressures, the individuality of gases begins to appear. An increase in external pressure and a decrease in temperature brings gas particles closer together, so intermolecular interaction begins to manifest itself to a greater extent. For such gases it is no longer possible to apply the Mendeleev-Clapeyron equation, but the van der Waals equation should be used:
where a and b are constant terms that take into account the presence of attractive forces between molecules and the molecules’ own volume, respectively. When gases are compressed, when there is a significant increase in their density, the forces MMV become more and more noticeable, which leads to the creation of conditions for the formation of various associates from molecules. Associates are relatively unstable groups of molecules. From the nature of the components MMV it follows that the universal interaction forces increase with increasing atomic sizes (polarizability increases sharply, therefore, the heavier the same type of particles (atoms or molecules) of a substance, the usually higher the degree of their association at a given temperature, the lower temperatures such a substance passes from gas to liquid .
IN liquids MMW forces are commensurate with the kinetic energy of molecular motion:
E MMV » E kin.
Therefore, the liquid has the property fluidity, takes the shape of the container in which it is placed. The peculiarity of the liquid structure is that there is no long-range order, but there is short-range order in the arrangement of molecules . The manifestation of short-range order is that molecules located in the 1st sphere of the environment of a given molecule linger to a greater extent near it and, thus, determine some order. However, even greater order with a long-range order element can appear in liquids. This occurs in cases where the universal forces of the IMV are complemented by the specific forces of the IMV. (hydrogen bond)
For solid state, the energy ratio is valid:
E MMV > E kin.
The solid state of matter is mainly found in the form of crystals. Crystals consist of particles (atoms, molecules, ions) of matter oriented in a certain way relative to each other. The nature of this orientation is such that substances that are sufficiently distant from the selected particle are in a strictly defined position and at a fixed distance. This property is called presence long-range order in crystals. Crystal shapes can be different.
15 A characteristic feature of the crystalline state is the presence systems of strictly ordered particles, which is called crystal lattice. A crystal lattice can be obtained by a certain movement (translation) in space of some smallest group of particles called unit cell.
The features of crystals are: a high degree of order (the presence of short- and long-range order), a certain symmetry of the elementary cells they form and, as a consequence, anisotropy (i.e., dependence on direction) of properties.
Depending on which particles lie at the nodes of the crystal lattice, ionic, atomic, molecular and metal lattices are distinguished.
Ionic lattice consists of ions of opposite sign alternating at the nodes. In this case, the ions can be simple (Na +, Cl - ..) and complex (NH 4 +, NO 3 - ...). Due to the fact that the ionic bond is unsaturated and non-directional, the ionic lattice is characterized by high coordination numbers(c.f. = 6.8) . Coordination number - the number of nearest particles surrounding the selected . Due to the high ionic bond strength, ionic crystal lattices are strong and their crystals have high melting points. Examples of compounds with ionic crystal lattices: NaCl, NH 4 NO 3, etc.
Atomic lattice consists of atoms connected by covalent bonds, for example, in diamond, graphite. The coordination numbers here are determined by the number of s-bonds of the central atom with surrounding atoms and do not reach large values (often about 4). Due to the high strength of the covalent bond, such lattices are very strong, and the substances are characterized by high melting points. It is known that diamond is the hardest natural substance.
Molecular lattice contains molecules in knots that are connected to each other due to intermolecular forces. Molecular lattices are low-strength, and substances with such lattices (solid H 2, O 2, N 2, CO 2, H 2 O) usually have low melting points.
Metal grate can be conventionally depicted in the form of positively charged ions located at nodes and electrons moving in interstices. The coordination number here reaches large values (8-12). The strength of the metal lattice varies widely and strongly depends on the presence of foreign impurities. In metals there is a chemical bond called metal bond. Basically, A metal bond is a special type of covalent bond. It arises as a result of the “massive” overlap of clouds of outer (valence) electrons of metal atoms.
16
One of the most essential properties of atoms of elements, which determine what kind of bond is formed between them - ionic or covalent, is Electronegativity
, i.e. the ability of atoms in a compound to attract electrons. A conditional quantitative assessment of electronegativity is given by the relative electronegativity scale. In periods there is a general tendency for the electronegativity of elements to increase, and in groups - for their decrease. Elements are arranged in a series according to their electronegativity, on the basis of which the electronegativity of elements located in different periods can be compared. The type of chemical bond depends on how large the difference in electronegativity values of the connecting atoms of the elements is. The more the atoms of the elements forming the bond differ in electronegativity, the more polar the chemical bond. Draw a sharp line between types
chemical bonds are not possible. In most compounds, the type of chemical bond is intermediate; for example, highly polar covalent
the chemical bond is close to an ionic bond. Depending on which of the limiting cases a chemical bond is closer in nature, it is classified as either an ionic or a covalent polar bond. Ionic bond
is an extreme case of a polarized covalent bond, when the shared electron pair belongs entirely to one of the atoms. In this case, a completely positive charge is realized on one of the atoms, and a completely negative charge on the other. This type of bond is characteristic of salts. For example, sodium chloride is NaCl. Each of the atoms contributes one electron to form a common electron pair. However, Cl completely displaces the resulting electron pair towards itself and thereby acquires a complete negative charge, and Na, which in this case does not have a single electron on the outer electronic level, has a complete positive charge. The most important differences between ionic bonds and other types of chemical bonds are lack of direction And unsaturation. That is why crystals formed due to ionic bonds gravitate towards various dense packings of the corresponding ions.
17 Spatial structure of molecules depends on the nature of the chemical bond that arises between atoms, and therefore, the structure of their electronic shell. Since s-, p-, d- and f-type electrons from each of the interacting atoms can participate in a chemical bond, the structure of the molecules depends on the type and number of electrons, as well as on the possibility of forming hybrid bonds. Often chemical bonds are formed by electrons located in different atomic orbitals (for example, s - and p - orbitals). Despite this, the connections turn out to be of equal value and are located symmetrically, which is ensured hybridization of atomic orbitals . Orbital hybridization is a change in the shape of some orbitals during the formation of a covalent bond to achieve more effective overlap of orbitals. As a result of hybridization, new hybrid orbitals appear, which are oriented in space in such a way that after their overlap with the orbitals of other atoms, the resulting electron pairs are as far apart as possible from each other friend. This minimizes the repulsion energy between electrons in the molecule. Hybridization is not a real process. This concept was introduced to describe the geometric structure of a molecule. The shape of particles resulting from the formation of covalent bonds involving hybrid atomic orbitals depends on the number and type of these orbitals. In this case, σ-bonds create a rigid “skeleton” of the particle:
Orbitals involved in hybridization. Type of hybridization Spatial shape of the molecule Examples
S,P sp – hybridization Linear BeCl2
s, p, p sp 2 – hybridization Triangular (flat trigonal) AlCl 3
s, p, p, p sp 3 – hybridization Tetrahedral CH 4
If electron clouds overlap along a line connecting the centers of atoms, then such a covalent bond is called sigma( )-connection
Covalent bond formed by lateral overlap R-orbitals of neighboring carbon atoms is called pi( )-connection.
